The Efficacy and Safety of a Qiliqiangxin Capsule Combined with Sacubitril/Valsartan in the Treatment of Chronic Heart Failure: A Systematic Review and Meta-Analysis
- PMID: 36777628
- PMCID: PMC9918363
- DOI: 10.1155/2023/2701314
The Efficacy and Safety of a Qiliqiangxin Capsule Combined with Sacubitril/Valsartan in the Treatment of Chronic Heart Failure: A Systematic Review and Meta-Analysis
Abstract
Background: Qiliqiangxin (QLQX) capsules are a commonly used proprietary Chinese medicine for the adjuvant treatment of chronic heart failure (CHF) in China. In recent years, several randomized controlled trials (RCTs) have reported on the efficacy and safety of QLQX combined with sacubitril/valsartan for CHF.
Objective: The purpose of this study was to systematically analyze the clinical efficacy and safety of QLQX combined with sacubitril/valsartan in the management of CHF and to provide clinicians as well as scientists with optimal evidence-based medical evidence.
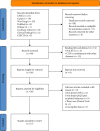
Methods: We searched RCTs to evaluate the efficacy and safety of QLQX combined with sacubitril/valsartan in the treatment of CHF in the Wanfang Database, China National Knowledge Infrastructure, China Science and Technology Journal Database, PubMed, Embase, and Cochrane Library databases from their inception until January 8, 2022. RCTs on QLQX in combination with sacubitril/valsartan for CHF were included. The outcome measures considered were total effective rate, left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVEDD), 6-minute walking distance (6-MWD), and adverse events. The quality of the included RCTs was assessed thereafter using the Cochrane risk of bias tool. RevMan 5.3 software was used to conduct the meta-analysis.
Results: The meta-analysis included 17 trials involving 1427 CHF patients. The results indicated that with sacubitril/valsartan administration combined with QLQX treatment, the total effective rate (relative risk (RR) = 1.24; 95% confidence interval (CI) (1.17, 1.31); p < 0.01), LVEF (mean difference (MD) = 6.20; 95% CI (5.36, 7.05; p < 0.01)), and 6-MWD (MD = 55.87; 95% CI (40.66, 71.09); p < 0.01) of CHF patients were significantly increased, and the LVEDD value of CHF patients was noted to be significantly reduced (MD = -3.98; 95% CI (-4.47, -3.48); p < 0.01). Moreover, there was no increase in the number of adverse events during treatment (RR = 0.67; 95% CI (0.33, 1.34); p < 0.01).
Conclusions: This study indicated that in CHF patients, on the basis of sacubitril/valsartan treatment, combination with QLQX can potentially enhance the total effective rate, improve LVEF and 6-MWD, and reduce LVEDD values, with good safety. However, considering the poor quality of the included studies, a multicenter, randomized, double-blind controlled study is needed for further confirmation.
Copyright © 2023 Wensheng Chen et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures






Similar articles
-
Qili Qiangxin Capsule Combined With Sacubitril/Valsartan for HFrEF: A Systematic Review and Meta-Analysis.Front Pharmacol. 2022 Apr 4;13:832782. doi: 10.3389/fphar.2022.832782. eCollection 2022. Front Pharmacol. 2022. PMID: 35444529 Free PMC article.
-
Sacubitril/Valsartan in the Treatment of Heart Failure With Reduced Ejection Fraction Focusing on the Impact on the Quality of Life: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.Cureus. 2023 Nov 11;15(11):e48674. doi: 10.7759/cureus.48674. eCollection 2023 Nov. Cureus. 2023. PMID: 38090453 Free PMC article. Review.
-
Meta-analysis of a controlled study of levosimendan combined with Sacubitril/Valsartan for the treatment of heart failure with reduced ejection fraction in China.Front Cardiovasc Med. 2024 Oct 3;11:1469457. doi: 10.3389/fcvm.2024.1469457. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39421155 Free PMC article.
-
Efficacy and Safety of Xinmailong Injection for Chronic Heart Failure: A Systematic Review and Meta-Analysis for Randomized Clinical Trials.J Integr Complement Med. 2025 Jun 6. doi: 10.1089/jicm.2025.0031. Online ahead of print. J Integr Complement Med. 2025. PMID: 40478767 Review.
-
Effect of sacubitril-valsartan on left ventricular remodeling in patients with acute myocardial infarction after primary percutaneous coronary intervention: a systematic review and meta-analysis.Front Pharmacol. 2024 May 28;15:1366035. doi: 10.3389/fphar.2024.1366035. eCollection 2024. Front Pharmacol. 2024. PMID: 38863978 Free PMC article.
Cited by
-
The traditional Chinese medicine Qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial.Nat Med. 2024 Aug;30(8):2295-2302. doi: 10.1038/s41591-024-03169-2. Epub 2024 Aug 2. Nat Med. 2024. PMID: 39095596 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources

