Mapping gene by early life stress interactions on child subcortical brain structures: A genome-wide prospective study
- PMID: 36777645
- PMCID: PMC7614163
- DOI: 10.1002/jcv2.12113
Mapping gene by early life stress interactions on child subcortical brain structures: A genome-wide prospective study
Abstract
Background: Although it is well-established that both genetics and the environment influence brain development, they are typically examined separately. Here, we aimed to prospectively investigate the interactive effects of genetic variants-from a genome-wide approach-and early life stress (ELS) on child subcortical brain structures, and their association with subsequent mental health problems.
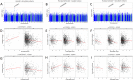
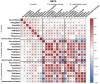
Method: Primary analyses were conducted using data from the Generation R Study (N = 2257), including genotype and cumulative prenatal and postnatal ELS scores (encompassing life events, contextual risk, parental risk, interpersonal risk, direct victimisation). Neuroimaging data were collected at age 10 years, including intracranial and subcortical brain volumes (accumbens, amygdala, caudate, hippocampus, pallidum, putamen, thalamus). Genome-wide association and genome-wide-by-environment interaction analyses (GWEIS, run separately for prenatal/postnatal ELS) were conducted for eight brain outcomes (i.e., 24 genome-wide analyses) in the Generation R Study (discovery). Polygenic scores (PGS) using the resulting weights were calculated in an independent (target) cohort (adolescent brain cognitive development Study; N = 10,751), to validate associations with corresponding subcortical volumes and examine links to later mother-reported internalising and externalising problems.
Results: One GWEIS-prenatal stress locus was associated with caudate volume (rs139505895, mapping onto PRSS12 and NDST3) and two GWEIS-postnatal stress loci with the accumbens (rs2397823 and rs3130008, mapping onto CUTA, SYNGAP1, and TABP). Functional annotation revealed that these genes play a role in neuronal plasticity and synaptic function, and have been implicated in neuro-developmental phenotypes, for example, intellectual disability, autism, and schizophrenia. None of these associations survived a more stringent correction for multiple testing across all analysis sets. In the validation sample, all PGSgenotype were associated with their respective brain volumes, but no PGSGxE associated with any subcortical volume. None of the PGS associated with internalising or externalising problems.
Conclusions: This study lends novel suggestive insights into gene-environment interplay on the developing brain as well as pointing to promising candidate loci for future replication and mechanistic studies.
Keywords: MRI; early life stress; gene-environment interaction; genome-wide association study; psychopathology.
Conflict of interest statement
Conflict of Interest Henning Tiemeier serves on the JCPP Advances Editorial Advisory Board. The remaining authors have declared that they have no competing or potential conflicts of interest.
Figures
Similar articles
-
Genetic Links Between Subcortical Brain Morphometry and Suicide Attempt Risk in Children and Adults.Hum Brain Mapp. 2025 May;46(7):e70220. doi: 10.1002/hbm.70220. Hum Brain Mapp. 2025. PMID: 40364472 Free PMC article.
-
Genetic scores for adult subcortical volumes associate with subcortical volumes during infancy and childhood.Hum Brain Mapp. 2021 Apr 15;42(6):1583-1593. doi: 10.1002/hbm.25292. Epub 2021 Feb 2. Hum Brain Mapp. 2021. PMID: 33528897 Free PMC article.
-
Trauma and posttraumatic stress disorder modulate polygenic predictors of hippocampal and amygdala volume.Transl Psychiatry. 2021 Dec 16;11(1):637. doi: 10.1038/s41398-021-01707-x. Transl Psychiatry. 2021. PMID: 34916497 Free PMC article.
-
Effects of Age and Sex on Subcortical Volumes.Front Aging Neurosci. 2019 Sep 26;11:259. doi: 10.3389/fnagi.2019.00259. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31616285 Free PMC article.
-
Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings.JAMA Psychiatry. 2015 May;72(5):490-9. doi: 10.1001/jamapsychiatry.2014.3162. JAMA Psychiatry. 2015. PMID: 25785435
Cited by
-
Positive or negative environmental modulations on human brain development: the morpho-functional outcomes of music training or stress.Front Neurosci. 2023 Nov 3;17:1266766. doi: 10.3389/fnins.2023.1266766. eCollection 2023. Front Neurosci. 2023. PMID: 38027483 Free PMC article. Review.
-
Prenatal stress alters mouse offspring dorsal striatal development and placental function in sex-specific ways.J Psychiatr Res. 2025 Feb;182:149-160. doi: 10.1016/j.jpsychires.2024.12.048. Epub 2025 Jan 1. J Psychiatr Res. 2025. PMID: 39809011
-
Mapping prenatal predictors and neurobehavioral outcomes of an epigenetic marker of neonatal inflammation - A longitudinal population-based study.Brain Behav Immun. 2024 Nov;122:483-496. doi: 10.1016/j.bbi.2024.08.053. Epub 2024 Aug 29. Brain Behav Immun. 2024. PMID: 39209009 Free PMC article.
References
-
- Acosta, H. , Kantojarvi, K. , Tuulari, J. J. , Lewis, J. D. , Hashempour, N. , Scheinin, N. M. , Lehtola, S. J. , Fonov, V. S. , Collins, D. L. , Evans, A. , Parkkola, R. , Lahdesmaki, T. , Saunavaara, J. , Merisaari, H. , Karlsson, L. , Paunio, T. , & Karlsson, H. (2020). Sex‐specific association between infant caudate volumes and a polygenic risk score for major depressive disorder. Journal of Neuroscience Research, 98(12), 2529–2540. 10.1002/jnr.24722 - DOI - PubMed
-
- Arango, C. , Dragioti, E. , Solmi, M. , Cortese, S. , Domschke, K. , Murray, R. M. , Jones, P. B. , Uher, R. , Carvalho, A. F. , Reichenberg, A. , Shin, J. I. , Andreassen, O. A. , Correll, C. U. , & Fusar‐Poli, P. (2021). Risk and protective factors for mental disorders beyond genetics: An evidence‐based atlas. World Psychiatry, 20(3), 417–436. 10.1002/wps.20894 - DOI - PMC - PubMed
-
- Autism Spectrum Disorders Working Group of the Psychiatric Genomics Consortium . (2017). Meta‐analysis of GWAS of over 16, 000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Molecular Autism, 8(1), 21. 10.1186/s13229-017-0137-9 - DOI - PMC - PubMed
-
- Bolhuis, K. , Tiemeier, H. , Jansen, P. R. , Muetzel, R. L. , Neumann, A. , Hillegers, M. H. J. , Van Den Akker, E. T. L. , Van Rossum, E. F. C. , Jaddoe, V. W. V. , Vernooij, M. W. , White, T. , & Kushner, S. A. (2019). Interaction of schizophrenia polygenic risk and cortisol level on pre‐adolescent brain structure. Psychoneuroendocrinology, 101, 295–303. 10.1016/j.psyneuen.2018.12.231 - DOI - PubMed
-
- Cai, N. , Revez, J. A. , Adams, M. J. , Andlauer, T. F. M. , Breen, G. , Byrne, E. M. , Clarke, T. K. , Forstner, A. J. , Grabe, H. J. , Hamilton, S. P. , Levinson, D. F. , Lewis, C. M. , Lewis, G. , Martin, N. G. , Milaneschi, Y. , Mors, O. , Muller‐Myhsok, B. , Penninx, B. , Perlis, R. H. , … Flint, J. (2020). Minimal phenotyping yields genome‐wide association signals of low specificity for major depression. Nature Genetics, 52(4), 437–447. 10.1038/s41588-020-0594-5 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
