Total Synthesis of (±)- N-Methyldibromoisophakellin and N-Methylugibohlin via Net [3+2] Cycloguanidinylations Employing 2-Amido-1,3-Diamino-Allyl Cations
- PMID: 36777739
- PMCID: PMC9914522
- DOI: 10.1016/j.tetlet.2022.154304
Total Synthesis of (±)- N-Methyldibromoisophakellin and N-Methylugibohlin via Net [3+2] Cycloguanidinylations Employing 2-Amido-1,3-Diamino-Allyl Cations
Abstract
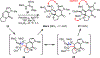
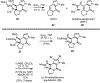
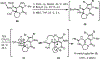
A concise total synthesis of (±)-N-methyldibromoisophakellin, a member of the monomeric pyrrole-aminoimidazole alkaloid family isolated from the marine sponge Stylissa carbica, was achieved via a net [3+2] cycloaddition to install the cyclic guanidine. This ring annulation employs a 2-amido-1,3-aminoallyl cation obtained under oxidative conditions from variously N-substituted guanidines which in one instance led to isolation of a tetracycle bearing a carbinolamine center through subsequent benzylic oxidation. Finally, the serendipitous formation of a unique, related alkenyl guanidine, N-methylugibohlin, achieved via ring opening of cyclic guanidine under acidic conditions suggests that ugibohlin may be an artifact of isolation.
Keywords: carbinolamine; guanidine; isolation artifact; pyrrole–aminoimidazole alkaloid; total synthesis.
Conflict of interest statement
Conflicts of Interest: The authors declare no competing financial interests. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Generation and Reactivity of 2-Amido-1,3-diaminoallyl Cations: Cyclic Guanidine Annulations via Net (3 + 2) and (4 + 3) Cycloadditions.Org Lett. 2020 Feb 21;22(4):1407-1413. doi: 10.1021/acs.orglett.0c00019. Epub 2020 Feb 3. Org Lett. 2020. PMID: 32009413 Free PMC article.
-
Synthesis and unexpected oxidization of the tricyclic core of ugibohlin, isophakellin, and styloguanidine.J Org Chem. 2013 Oct 18;78(20):10459-68. doi: 10.1021/jo401911a. Epub 2013 Oct 3. J Org Chem. 2013. PMID: 24050807
-
A likely biogenetic gateway linking 2-aminoimidazolinone metabolites of sponges to proline: spontaneous oxidative conversion of the pyrrole-proline-guanidine pseudo-peptide to dispacamide A.J Am Chem Soc. 2004 Aug 25;126(33):10252-3. doi: 10.1021/ja047574e. J Am Chem Soc. 2004. PMID: 15315431
-
[Total synthesis of marine cyclic guanidine compounds and development of novel guanidine type asymmetric organocatalysts].Yakugaku Zasshi. 2003 Jun;123(6):387-98. doi: 10.1248/yakushi.123.387. Yakugaku Zasshi. 2003. PMID: 12822483 Review. Japanese.
-
Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids.Nat Prod Rep. 2011 Jul;28(7):1229-60. doi: 10.1039/c0np00013b. Epub 2011 May 9. Nat Prod Rep. 2011. PMID: 21556392 Free PMC article. Review.
References
-
- Hoffmann H; Lindel T Synthesis of the Pyrrole-Imidazole Alkaloids. Synthesis 2003, 1753–1783. DOI: 10.1055/s-2003-41005. - DOI
- Forte B; Malgesini B; Piutti C; Quartieri F; Scolaro A; Papeo G A Submarine Journey: The Pyrrole-Imidazole Alkaloids. Mar. Drugs 2009, 7, 705–753. DOI: 10.3390/md7040705. - DOI - PMC - PubMed
- Al-Mourabit A; Zancanella MA; Tilvi S; Romo D Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. DOI: 10.1039/c0np00013b. - DOI - PMC - PubMed
- Beniddir MA; Evanno L; Joseph D; Skiredj A; Poupon E Emergence of diversity and stereochemical outcomes in the biosynthetic pathways of cyclobutane-centered marine alkaloid dimers. Nat. Prod. Rep. 2016, 33, 820–842. DOI: 10.1039/c5np00159e. - DOI - PubMed
- Seipp K; Geske L; Opatz T Marine Pyrrole Alkaloids. Mar. Drugs 2021, 19, 514. DOI: 10.3390/md19090514. - DOI - PMC - PubMed
- Herath AK; Lovely CJ Pyrrole carboxamide introduction in the total synthesis of pyrrole–imidazole alkaloids. Org. Biomol. Chem. 2021, 19, 2603–2621. DOI: 10.1039/d0ob02052d. - DOI - PubMed
-
- Burkholder PR; Sharma GM Antimicrobial agents from the sea. Lloydia 1969, 32, 466–483. - PubMed
- Sharma GM; Burkholder PR Structure of Dibromophakellin, a New Bromine-containing Alkaloid from the Marine Sponge Phakellia flabellata. J. Chem. Soc. Chem. D 1971, 151–152, 10.1039/C29710000151. DOI: 10.1039/C29710000151. - DOI - DOI
- Sharma GM; Magdoff-Fairchild B Natural products of marine sponges. 7. The constitution of weakly basic guanidine compounds, dibromophakellin and monobromophakellin. J. Org. Chem. 1977, 42, 4118–4124. DOI: 10.1021/jo00445a028. - DOI
-
- Kinnel RB; Gehrken H-P; Swali R; Skoropowski G; Scheuer PJ Palau’amine and Its Congeners: A Family of Bioactive Bisguanidines from the Marine Sponge Stylotella aurantium1. J. Org. Chem. 1998, 63, 3281–3286. DOI: 10.1021/jo971987z. - DOI
- Kinnel RB; Gehrken HP; Scheuer PJ Palau’amine: a cytotoxic and immunosuppressive hexacyclic bisguanidine antibiotic from the sponge Stylotella agminata. J. Am. Chem. Soc. 2002, 115, 3376–3377. DOI: 10.1021/ja00061a065. - DOI
- Buchanan MS; Carroll AR; Quinn RJ Revised structure of palau’amine. Tetrahedron Lett. 2007, 48, 4573–4574. DOI: 10.1016/j.tetlet.2007.04.128. - DOI
-
- Grube A; Kock M Stylissadines A and B: the first tetrameric pyrrole-imidazole alkaloids. Org. Lett. 2006, 8, 4675–4678. DOI: 10.1021/ol061317s. - DOI - PubMed
- Buchanan MS; Carroll AR; Addepalli R; Avery VM; Hooper JN; Quinn RJ Natural products, stylissadines A and B, specific antagonists of the P2X7 receptor, an important inflammatory target. J. Org. Chem. 2007, 72, 2309–2317. DOI: 10.1021/jo062007q. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

