Total Synthesis of (±)- N-Methyldibromoisophakellin and N-Methylugibohlin via Net [3+2] Cycloguanidinylations Employing 2-Amido-1,3-Diamino-Allyl Cations
- PMID: 36777739
- PMCID: PMC9914522
- DOI: 10.1016/j.tetlet.2022.154304
Total Synthesis of (±)- N-Methyldibromoisophakellin and N-Methylugibohlin via Net [3+2] Cycloguanidinylations Employing 2-Amido-1,3-Diamino-Allyl Cations
Abstract
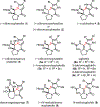
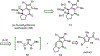
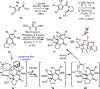
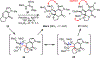
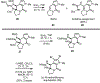
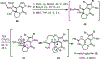
A concise total synthesis of (±)-N-methyldibromoisophakellin, a member of the monomeric pyrrole-aminoimidazole alkaloid family isolated from the marine sponge Stylissa carbica, was achieved via a net [3+2] cycloaddition to install the cyclic guanidine. This ring annulation employs a 2-amido-1,3-aminoallyl cation obtained under oxidative conditions from variously N-substituted guanidines which in one instance led to isolation of a tetracycle bearing a carbinolamine center through subsequent benzylic oxidation. Finally, the serendipitous formation of a unique, related alkenyl guanidine, N-methylugibohlin, achieved via ring opening of cyclic guanidine under acidic conditions suggests that ugibohlin may be an artifact of isolation.
Keywords: carbinolamine; guanidine; isolation artifact; pyrrole–aminoimidazole alkaloid; total synthesis.
Conflict of interest statement
Conflicts of Interest: The authors declare no competing financial interests. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
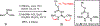
References
-
- Hoffmann H; Lindel T Synthesis of the Pyrrole-Imidazole Alkaloids. Synthesis 2003, 1753–1783. DOI: 10.1055/s-2003-41005. - DOI
- Forte B; Malgesini B; Piutti C; Quartieri F; Scolaro A; Papeo G A Submarine Journey: The Pyrrole-Imidazole Alkaloids. Mar. Drugs 2009, 7, 705–753. DOI: 10.3390/md7040705. - DOI - PMC - PubMed
- Al-Mourabit A; Zancanella MA; Tilvi S; Romo D Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. DOI: 10.1039/c0np00013b. - DOI - PMC - PubMed
- Beniddir MA; Evanno L; Joseph D; Skiredj A; Poupon E Emergence of diversity and stereochemical outcomes in the biosynthetic pathways of cyclobutane-centered marine alkaloid dimers. Nat. Prod. Rep. 2016, 33, 820–842. DOI: 10.1039/c5np00159e. - DOI - PubMed
- Seipp K; Geske L; Opatz T Marine Pyrrole Alkaloids. Mar. Drugs 2021, 19, 514. DOI: 10.3390/md19090514. - DOI - PMC - PubMed
- Herath AK; Lovely CJ Pyrrole carboxamide introduction in the total synthesis of pyrrole–imidazole alkaloids. Org. Biomol. Chem. 2021, 19, 2603–2621. DOI: 10.1039/d0ob02052d. - DOI - PubMed
-
- Burkholder PR; Sharma GM Antimicrobial agents from the sea. Lloydia 1969, 32, 466–483. - PubMed
- Sharma GM; Burkholder PR Structure of Dibromophakellin, a New Bromine-containing Alkaloid from the Marine Sponge Phakellia flabellata. J. Chem. Soc. Chem. D 1971, 151–152, 10.1039/C29710000151. DOI: 10.1039/C29710000151. - DOI - DOI
- Sharma GM; Magdoff-Fairchild B Natural products of marine sponges. 7. The constitution of weakly basic guanidine compounds, dibromophakellin and monobromophakellin. J. Org. Chem. 1977, 42, 4118–4124. DOI: 10.1021/jo00445a028. - DOI
-
- Kinnel RB; Gehrken H-P; Swali R; Skoropowski G; Scheuer PJ Palau’amine and Its Congeners: A Family of Bioactive Bisguanidines from the Marine Sponge Stylotella aurantium1. J. Org. Chem. 1998, 63, 3281–3286. DOI: 10.1021/jo971987z. - DOI
- Kinnel RB; Gehrken HP; Scheuer PJ Palau’amine: a cytotoxic and immunosuppressive hexacyclic bisguanidine antibiotic from the sponge Stylotella agminata. J. Am. Chem. Soc. 2002, 115, 3376–3377. DOI: 10.1021/ja00061a065. - DOI
- Buchanan MS; Carroll AR; Quinn RJ Revised structure of palau’amine. Tetrahedron Lett. 2007, 48, 4573–4574. DOI: 10.1016/j.tetlet.2007.04.128. - DOI
-
- Grube A; Kock M Stylissadines A and B: the first tetrameric pyrrole-imidazole alkaloids. Org. Lett. 2006, 8, 4675–4678. DOI: 10.1021/ol061317s. - DOI - PubMed
- Buchanan MS; Carroll AR; Addepalli R; Avery VM; Hooper JN; Quinn RJ Natural products, stylissadines A and B, specific antagonists of the P2X7 receptor, an important inflammatory target. J. Org. Chem. 2007, 72, 2309–2317. DOI: 10.1021/jo062007q. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

