Kinetic study of polydopamine sphere synthesis using TRIS: relationship between synthesis conditions and final properties
- PMID: 36777934
- PMCID: PMC9909370
- DOI: 10.1039/d2ra06669f
Kinetic study of polydopamine sphere synthesis using TRIS: relationship between synthesis conditions and final properties
Abstract
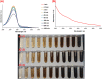
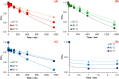
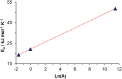
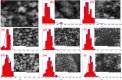
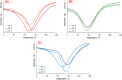
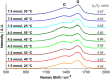
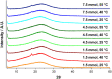
The synthesis and characterization of polydopamine (PDA) using dopamine (DA) as the monomer and (hydroxymethyl)aminomethane (TRIS) as the oxidant is studied. The effect of temperature and TRIS concentration on the kinetics of dopamine polymerization is evaluated, and the kinetic parameters are also calculated. Three TRIS concentrations are used to assess their effect on DA polymerization kinetics. The reaction at 1.5 mmol of TRIS shows a sustained increase of the rate constant with temperature from 2.38 × 10-4 to 5.10 × 10-4 when the temperature is increased from 25 to 55 °C; however, not all reactions follow an Arrhenius law. In addition, the correlation between the synthesis parameters and morphological, structural, and thermal properties of polydopamine is established. The morphology of the PDA particles is evaluated by Scanning Electron Microscopy (SEM), the relationships between the diameter, distribution size, and the rate constant. Thermal characterization by Differential Scanning Calorimetry (DSC) shows an endothermic transition around 130 °C associated with the melting of PDA's regular structure. It is supported by structural studies, such as infrared and Raman spectroscopy and X-ray Diffraction (XRD), by observing a broad peak at 23.1° (2θ) that fits with a graphitic-like structure of PDA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Ito S. Wakamatsu K. Quantitative Analysis of Eumelanin and Pheomelanin in Humans, Mice, and Other Animals: a Comparative Review. Pigm. Cell Res. 2003;16:523–531. - PubMed
-
- Ligonzo T. et al., Electrical and optical properties of natural and synthetic melanin biopolymer. J. Non-Cryst. Solids. 2009;355:1221–1226.
-
- Abbas M. et al., Structural, electrical, electronic and optical properties of melanin films. Eur. Phys. J. E: Soft Matter Biol. Phys. 2009;28:285–291. - PubMed
-
- Postma A. et al., Self-Polymerization of Dopamine as a Versatile and Robust Technique to Prepare Polymer Capsules. Chem. Mater. 2009;21:3042–3044.
LinkOut - more resources
Full Text Sources
Other Literature Sources

