Electrochemical stripping voltammetrical sensor based on polypyrrole exfoliated polyetheramine-montmorillonite nanocomposite for nanomolar detection of nifuroxazide
- PMID: 36777946
- PMCID: PMC9909373
- DOI: 10.1039/d2ra06160k
Electrochemical stripping voltammetrical sensor based on polypyrrole exfoliated polyetheramine-montmorillonite nanocomposite for nanomolar detection of nifuroxazide
Abstract
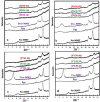
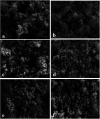
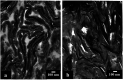
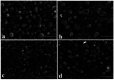
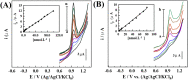
A series of polypyrrole/polyetheramine-montmorillonite nanocomposites have been fabricated by the intercalation of different types of polyoxyalkylene amine hydrochloride (Jeffamines: D400, D2000, T5000, and T403) into montmorillonite layers via the cation-exchange process followed by in situ polymerization of pyrrole. The physicochemical characteristics of as-prepared nanocomposites were investigated using Fourier transform infrared (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET), and electrochemical impedance spectroscopy (EIS) instruments. The change of the types of Jeffamine causes a change in the geometrical structure, and surface area of nanocomposites. Noteworthy, the resulting polypyrrole/D2000-montmorillonite ([PDM-50]) nanocomposite exhibited a cauliflower-like shape with a specific surface area (116.2 m2 g-1) with the highest conductivity. Furthermore, the modified stripping voltammetric carbon paste sensor was fabricated based on 1.0% [PDM-50] nanocomposites to detect the drug nifuroxazide (NF). The sensor achieved detection limits (LD) of 0.24, and 0.9 nM of NF in the medication, and human urine fluid, respectively. This sensor showed appropriate repeatability, reproducibility, stability, and selectivity for NF sensing in different fluids accompanied by other interferents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Green fabrication of 3D hierarchical blossom-like hybrid of peeled montmorillonite-ZnO for in-vitro electrochemical sensing of diltiazem hydrochloride drug.Mater Sci Eng C Mater Biol Appl. 2020 Jun;111:110773. doi: 10.1016/j.msec.2020.110773. Epub 2020 Feb 26. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32279745
-
Ultra-sensitive detection of 4-chloro-2-methylphenoxyacetic acid herbicide using a porous Co-1,4-benzenedicarboxylate /montmorillonite nanocomposite sensor.Mikrochim Acta. 2024 Dec 24;192(1):30. doi: 10.1007/s00604-024-06765-8. Mikrochim Acta. 2024. PMID: 39718606 Free PMC article.
-
Synthesis and validation of ultrasensitive stripping voltammetric sensor based on polypyrrole@ZnO/Fe3O4 core-shell nanostructure for picomolar detection of artesunate and dopamine drugs.Anal Methods. 2022 Oct 6;14(38):3739-3750. doi: 10.1039/d2ay00864e. Anal Methods. 2022. PMID: 36124547
-
Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode.Ultrason Sonochem. 2019 Jul;55:196-206. doi: 10.1016/j.ultsonch.2019.01.038. Epub 2019 Jan 29. Ultrason Sonochem. 2019. PMID: 30878204 Review.
-
Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review.Anal Chim Acta. 2013 Jul 30;789:1-16. doi: 10.1016/j.aca.2013.04.021. Epub 2013 Apr 29. Anal Chim Acta. 2013. PMID: 23856225 Review.
References
-
- Greenstein G. R. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals Ref. Rev. 2007;21:40.
-
- Maher H. M. Belal T. S. J. Liq. Chromatogr. Relat. Technol. 2012;35:2001–2020.
-
- Belal T. S. A simple and sensitive spectrofluorimetric method for analysis of some nitrofuran drugs in pharmaceutical preparations. J. Fluoresc. 2008;18:771–780. - PubMed
-
- Abul Khier A. Elhenawee M. M. Elmasry M. S. Spectrophotometric method for the determination of some drugs using fast red B salt. J. Chem. 2008;5:1087–1097.
LinkOut - more resources
Full Text Sources
Research Materials

