Preferences of Adult Patients With Inflammatory Bowel Disease for Attributes of Clinical Trials: Evidence From a Choice-Based Conjoint Analysis
- PMID: 36777964
- PMCID: PMC9802344
- DOI: 10.1093/crocol/otz048
Preferences of Adult Patients With Inflammatory Bowel Disease for Attributes of Clinical Trials: Evidence From a Choice-Based Conjoint Analysis
Abstract
Background: Clinical trial recruitment is the rate-limiting step in developing new treatments. To understand inflammatory bowel disease (IBD) patient recruitment, we investigated two questions: Do changes in clinical trial attributes, like monetary compensation, influence recruitment rates, and does this influence differ across subgroups?
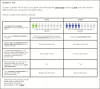
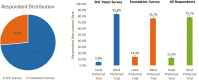
Methods: We answered these questions through a conjoint survey of 949 adult IBD patients.
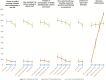
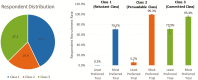
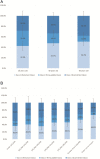
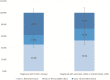
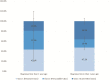
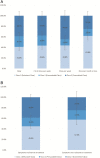
Results: Recruitment rates are influenced by trial attributes: small but significant increases are predicted with lower placebo rates, reduced number of endoscopies, less time commitment, open label extension, and increased involvement of participant's primary GI physician. A much stronger effect was found with increased monetary compensation. Latent class analysis indicated three patient subgroups: some patients quite willing to participate in IBD trials, some quite reluctant, and others who can be persuaded. The persuadable group is quite sensitive to monetary compensation, and payments up to US$2,000 for a 1-year study could significantly increase recruitment rates for IBD clinical trials.
Conclusions: This innovative study provides researchers with a framework for predicting recruitment rates for different IBD clinical trials.
Keywords: Crohn’s disease; clinical trials; conjoint analysis; inflammatory bowel disease; patient recruitment; ulcerative colitis.
© 2019 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Figures



References
-
- Dahlhamer JM. Prevalence of inflammatory bowel disease among adults aged≥ 18 years—United States, 2015. MMWR. 2016;65(42):1166–1169. https://www.cdc.gov/mmwr/volumes/65/wr/mm6542a3.htm. Accessed July 11, 2019. - PubMed
-
- Ehrlich O, Testaverde J, Heller C, et al. . Crohn’s disease and ulcerative colitis patient perspectives on clinical trials and participation. medRxiv. 2018;19000273. Accessed July 11, 2019.
-
- Getz KA. Enrollment performance: weighing the facts. Appl Clin Trials. 2012; 21(5). http://www.appliedclinicaltrialsonline.com/enrollment-performance-weighi.... Accessed July 12, 2019.
-
- Roan S. Medical Clinical Research Slows for Lack of Patients. California:Los Angeles Times. March 14, 2009. http://articles.latimes.com/2009/mar/14/science/sci-clinical-trials14. Accessed July 11, 2019.
-
- Anderson A, Getz KA. Insights and best practices for planning and implementing patient advisory boards. Ther Innov Regul Sci. 2018;52:469–473. - PubMed
LinkOut - more resources
Full Text Sources
