Interaction of copper potential metallodrugs with TMPRSS2: A comparative study of docking tools and its implications on COVID-19
- PMID: 36778030
- PMCID: PMC9909424
- DOI: 10.3389/fchem.2023.1128859
Interaction of copper potential metallodrugs with TMPRSS2: A comparative study of docking tools and its implications on COVID-19
Abstract
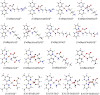
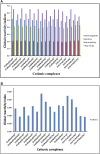
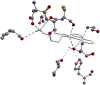
SARS-CoV-2 is the virus responsible for the COVID-19 pandemic. For the virus to enter the host cell, its spike (S) protein binds to the ACE2 receptor, and the transmembrane protease serine 2 (TMPRSS2) cleaves the binding for the fusion. As part of the research on COVID-19 treatments, several Casiopeina-analogs presented here were looked at as TMPRSS2 inhibitors. Using the DFT and conceptual-DFT methods, it was found that the global reactivity indices of the optimized molecular structures of the inhibitors could be used to predict their pharmacological activity. In addition, molecular docking programs (AutoDock4, Molegro Virtual Docker, and GOLD) were used to find the best potential inhibitors by looking at how they interact with key amino acid residues (His296, Asp 345, and Ser441) in the catalytic triad. The results show that in many cases, at least one of the amino acids in the triad is involved in the interaction. In the best cases, Asp435 interacts with the terminal nitrogen atoms of the side chains in a similar way to inhibitors such as nafamostat, camostat, and gabexate. Since the copper compounds localize just above the catalytic triad, they could stop substrates from getting into it. The binding energies are in the range of other synthetic drugs already on the market. Because serine protease could be an excellent target to stop the virus from getting inside the cell, the analyzed complexes are an excellent place to start looking for new drugs to treat COVID-19.
Keywords: COVID-19; Casiopeina analogs; Casiopeina-like metallodrugs; DFT; TMPRSS2; copper; molecular docking.
Copyright © 2023 Vazquez-Rodriguez, Ramírez-Contreras, Noriega, García-García, Sánchez-Gaytán, Melendez, Castro, de Azevedo and González-Vergara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Structural insights and inhibition mechanism of TMPRSS2 by experimentally known inhibitors Camostat mesylate, Nafamostat and Bromhexine hydrochloride to control SARS-coronavirus-2: A molecular modeling approach.Inform Med Unlocked. 2021;24:100597. doi: 10.1016/j.imu.2021.100597. Epub 2021 May 26. Inform Med Unlocked. 2021. PMID: 34075338 Free PMC article.
-
Targeting mechanism for SARS-CoV-2 in silico: interaction and key groups of TMPRSS2 toward four potential drugs.Nanoscale. 2021 Nov 25;13(45):19218-19237. doi: 10.1039/d1nr06313h. Nanoscale. 2021. PMID: 34787160
-
The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19.mBio. 2021 Aug 31;12(4):e0097021. doi: 10.1128/mBio.00970-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340553 Free PMC article.
-
Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies.Eur J Clin Pharmacol. 2020 Dec;76(12):1623-1630. doi: 10.1007/s00228-020-02963-4. Epub 2020 Jul 21. Eur J Clin Pharmacol. 2020. PMID: 32696234 Free PMC article. Review.
-
Targeting Host Cell Proteases to Prevent SARS-CoV-2 Invasion.Curr Drug Targets. 2021;22(2):192-201. doi: 10.2174/1389450121666200924113243. Curr Drug Targets. 2021. PMID: 32972339 Review.
Cited by
-
L-Citrullinato-Bipyridine and L-Citrullinato-Phenanthroline Mixed Copper Complexes: Synthesis, Characterization and Potential Anticancer Activity.Pharmaceutics. 2024 May 31;16(6):747. doi: 10.3390/pharmaceutics16060747. Pharmaceutics. 2024. PMID: 38931869 Free PMC article.
References
-
- Adamo C., Barone V. (1998). Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The MPW and MPW1PW models. J. Chem. Phys. 108, 664–675. 10.1063/1.475428 - DOI
-
- Adelusi T. I., Oyedele A.-Q. K., Boyenle I. D., Ogunlana A. T., Adeyemi R. O., Ukachi C. D., et al. (2022). Molecular modeling in drug discovery. Inf. Med. Unlocked 29, 100880. 10.1016/j.imu.2022.100880 - DOI
-
- Al-Harbi S. A. (2022). Synthesis and characterization of violurate-based Mn(II) and Cu(II) complexes nano-crystallites as DNA-binders and therapeutics agents against SARS-CoV-2 virus. J. Saudi Chem. Soc. 26, 101528. 10.1016/j.jscs.2022.101528 - DOI
-
- Aprajita, Choudhary M. (2022). Structural and computational studies of cobalt(II) and copper(II) complexes with aromatic heterocyclic ligand. Polycycl. Aromat. Compd. 42, 1–27. 10.1080/10406638.2022.2061530 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

