H2S-based fluorescent imaging for pathophysiological processes
- PMID: 36778034
- PMCID: PMC9911449
- DOI: 10.3389/fchem.2023.1126309
H2S-based fluorescent imaging for pathophysiological processes
Abstract
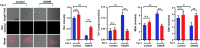
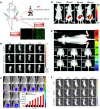
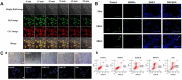
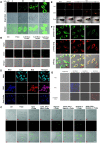
Hydrogen sulfide (H2S), as an important endogenous signaling molecule, plays a vital role in many physiological processes. The abnormal behaviors of hydrogen sulfide in organisms may lead to various pathophysiological processes. Monitoring the changes in hydrogen sulfide is helpful for pre-warning and treating these pathophysiological processes. Fluorescence imaging techniques can be used to observe changes in the concentration of analytes in organisms in real-time. Therefore, employing fluorescent probes imaging to investigate the behaviors of hydrogen sulfide in pathophysiological processes is vital. This paper reviews the design strategy and sensing mechanisms of hydrogen sulfide-based fluorescent probes, focusing on imaging applications in various pathophysiological processes, including neurodegenerative diseases, inflammation, apoptosis, oxidative stress, organ injury, and diabetes. This review not only demonstrates the specific value of hydrogen sulfide fluorescent probes in preclinical studies but also illuminates the potential application in clinical diagnostics.
Keywords: biomarker; fluorescence probe; hydrogen sulfide; pathophysiological processes; visualization.
Copyright © 2023 Jia, Zhang, Hou, Niu and Wang.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

