Regulating Neutrophil PAD4/NOX-Dependent Cerebrovasular Thromboinflammation
- PMID: 36778112
- PMCID: PMC9910005
- DOI: 10.7150/ijbs.77434
Regulating Neutrophil PAD4/NOX-Dependent Cerebrovasular Thromboinflammation
Abstract
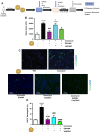
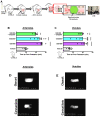
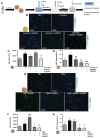
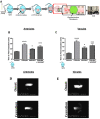
Background: Neutrophil extracellular trap (NET) production has been implicated in the pathogenesis of thromboinflammatory conditions such as Sickle Cell Disease (SCD), contributing to heightened risk for ischemic stroke. NETs are catalyzed by the enzyme Peptidyl Arginine Deiminase 4 (PAD4) and neutrophil derived reactive oxygen species (ROS), especially NADPH oxidase (NOX) which interacts with PAD4 and is therefore critical for neutrophil function. However, the role that NOX-dependent ROS and NETs play in the accelerated cerebral microvascular thrombosis associated with thromboinflammatory conditions, such as SCD, has not been fully elucidated and is the aim of this study. Methods: The in-vitro effects of targeting PAD4 and NOX were examined using physiologically relevant NET assays with neutrophils isolated from healthy volunteers (control) and SCD patients. In addition, in-vivo intravascular effects of targeting PAD4 and NOX in the cerebral microcirculation of C57BL/6 and sickle transgenic mice (STM) were assessed using a photoactivation thrombosis model (light/dye) coupled with real-time fluorescence intravital microscopy. Results: We found that targeting PAD4 and NOX in human neutrophils significantly inhibited ionomycin dependent H3cit+ neutrophils. Targeting PAD4 and NOX in-vivo resulted in prolonged blood flow cessation in cerebrovascular arterioles as well as venules. Moreover, we were able to replicate the effects of PAD4 and NOX targeting in a clinical model of accelerated thromboinflammation by increasing blood flow cessation times in cerebral microvessels in STM. These findings concurred with the clinical setting i.e. neutrophils isolated from SCD patients, which possessed an attenuation of H3cit+ neutrophil production on targeting PAD4 and NOX. Conclusions: Taken together, our compelling data suggests that PAD4 and NOX play a significant role in neutrophil driven thromboinflammation. Targeting PAD4 and NOX limits pathological H3cit+ neutrophils, which may further explain attenuation of cerebral thrombosis. Overall, this study presents a viable pre-clinical model of prevention and management of thromboinflammatory complications such as ischemic stroke.
Keywords: NADPH oxidase; Thromboinflammation; neutrophil extracellular traps; neutrophils; peptidyl arginine deiminase 4; thrombosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and β-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3.Front Immunol. 2018 May 29;9:1182. doi: 10.3389/fimmu.2018.01182. eCollection 2018. Front Immunol. 2018. PMID: 29896200 Free PMC article.
-
Viral mimetic poly(I:C) induces neutrophil extracellular traps via PAD4 to promote inflammation and thrombosis.Biochem Biophys Res Commun. 2021 Aug 6;565:64-71. doi: 10.1016/j.bbrc.2021.05.091. Epub 2021 Jun 4. Biochem Biophys Res Commun. 2021. PMID: 34098313
-
β-Conglycinin induces the formation of neutrophil extracellular traps dependent on NADPH oxidase-derived ROS, PAD4, ERK1/2 and p38 signaling pathways in mice.Food Funct. 2021 Jan 7;12(1):154-161. doi: 10.1039/d0fo02337j. Epub 2020 Dec 8. Food Funct. 2021. PMID: 33289753
-
Thromboinflammation: From Atherosclerosis to COVID-19.Arterioscler Thromb Vasc Biol. 2022 Sep;42(9):1103-1112. doi: 10.1161/ATVBAHA.122.317162. Epub 2022 Jul 8. Arterioscler Thromb Vasc Biol. 2022. PMID: 35861953 Free PMC article. Review.
-
To Gain Insights into the Pathophysiological Mechanisms of the Thrombo-Inflammatory Process in the Atherosclerotic Plaque.Int J Mol Sci. 2023 Dec 19;25(1):47. doi: 10.3390/ijms25010047. Int J Mol Sci. 2023. PMID: 38203218 Free PMC article. Review.
Cited by
-
The role of extracellular traps released by neutrophils, eosinophils, and macrophages in asthma.Respir Res. 2024 Jul 30;25(1):290. doi: 10.1186/s12931-024-02923-x. Respir Res. 2024. PMID: 39080638 Free PMC article. Review.
-
Immunothrombosis: A bibliometric analysis from 2003 to 2023.Medicine (Baltimore). 2024 Sep 13;103(37):e39566. doi: 10.1097/MD.0000000000039566. Medicine (Baltimore). 2024. PMID: 39287275 Free PMC article.
-
Reducing Abdominal Aortic Aneurysm Progression by Blocking Neutrophil Extracellular Traps Depends on Thrombus Formation.JACC Basic Transl Sci. 2024 Jan 10;9(3):342-360. doi: 10.1016/j.jacbts.2023.11.003. eCollection 2024 Mar. JACC Basic Transl Sci. 2024. PMID: 38559632 Free PMC article.
-
Neutrophil extracellular traps mediate neuro-immunothrombosis.Neural Regen Res. 2024 Aug 1;19(8):1734-1740. doi: 10.4103/1673-5374.389625. Epub 2023 Dec 11. Neural Regen Res. 2024. PMID: 38103239 Free PMC article.
-
Mast cell extracellular trap formation underlies vascular and neural injury and hyperalgesia in sickle cell disease.Life Sci Alliance. 2024 Sep 6;7(11):e202402788. doi: 10.26508/lsa.202402788. Print 2024 Nov. Life Sci Alliance. 2024. PMID: 39242155 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

