An Overview: The Diversified Role of Mitochondria in Cancer Metabolism
- PMID: 36778129
- PMCID: PMC9910000
- DOI: 10.7150/ijbs.81609
An Overview: The Diversified Role of Mitochondria in Cancer Metabolism
Abstract
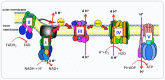
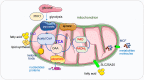
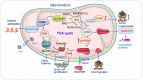
Mitochondria are intracellular organelles involved in energy production, cell metabolism and cell signaling. They are essential not only in the process of ATP synthesis, lipid metabolism and nucleic acid metabolism, but also in tumor development and metastasis. Mutations in mtDNA are commonly found in cancer cells to promote the rewiring of bioenergetics and biosynthesis, various metabolites especially oncometabolites in mitochondria regulate tumor metabolism and progression. And mutation of enzymes in the TCA cycle leads to the unusual accumulation of certain metabolites and oncometabolites. Mitochondria have been demonstrated as the target for cancer treatment. Cancer cells rely on two main energy resources: oxidative phosphorylation (OXPHOS) and glycolysis. By manipulating OXPHOS genes or adjusting the metabolites production in mitochondria, tumor growth can be restrained. For example, enhanced complex I activity increases NAD+/NADH to prevent metastasis and progression of cancers. In this review, we discussed mitochondrial function in cancer cell metabolism and specially explored the unique role of mitochondria in cancer stem cells and the tumor microenvironment. Targeting the OXPHOS pathway and mitochondria-related metabolism emerging as a potential therapeutic strategy for various cancers.
Keywords: cancer; mitochondria; tumor metabolism; tumor metastasis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clinical Cancer Research. 2018;24:2482–90. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

