Diabetes-Induced Autophagy Dysregulation Engenders Testicular Impairment via Oxidative Stress
- PMID: 36778206
- PMCID: PMC9918358
- DOI: 10.1155/2023/4365895
Diabetes-Induced Autophagy Dysregulation Engenders Testicular Impairment via Oxidative Stress
Abstract
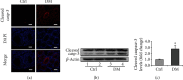
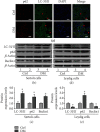
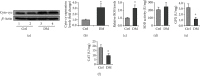
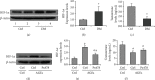
Testes produce sperms, and gamete generation relies on a proper niche environment. The disruption of hierarchical regulatory homeostasis in Leydig or Sertoli cells may evoke a sterile phenotype in humans. In this study, we recapitulated type 2 diabetes mellitus by using a high-fat diet- (HFD-) fed mouse model to identify the phenotype and potential mechanism of diabetes-induced testicular impairment. At the end of the study, blood glucose levels, testosterone structure, testicular antioxidant capacity, and testosterone level and the expression of hypoxia-inducible factor- (HIF-) 1α, apoptosis-related protein cleaved-caspase3, and autophagy-related proteins such as LC3I/II, p62, and Beclin1 were evaluated. We found that long-term HFD treatment causes the development of diabetes mellitus, implicating increased serum glucose level, cell apoptosis, and testicular atrophy (P < 0.05 vs. Ctrl). Mechanistically, the results showed enhanced expression of HIF-1α in both Sertoli and Leydig cells (P < 0.05 vs. Ctrl). Advanced glycation end products (AGEs) were demonstrated to be a potential factor leading to HIF-1α upregulation in both cell types. In Sertoli cells, high glucose treatment had minor effects on Sertoli cell autophagy. However, AGE treatment stagnated the autophagy flux and escalated cell apoptosis (P < 0.05 vs. Ctrl+Ctrl). In Leydig cells, high glucose treatment was adequate to encumber autophagy induction and enhance oxidative stress. Similarly, AGE treatment facilitated HIF-1α expression and hampered testosterone production (P < 0.05 vs. Ctrl+Ctrl). Overall, these findings highlight the dual effects of diabetes on autophagy regulation in Sertoli and Leydig cells while imposing oxidative stress in both cell types. Furthermore, the upregulation of HIF-1α, which could be triggered by AGE treatment, may negatively affect both cell types. Together, these findings will help us further understand the molecular mechanism of diabetes-induced autophagy dysregulation and testicular impairment, enriching the content of male reproductive biology in diabetic patients.
Copyright © 2023 Renfeng Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
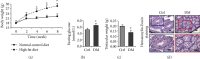
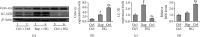



Similar articles
-
The role of HIF-1α-mediated autophagy in ionizing radiation-induced testicular injury.J Mol Histol. 2023 Oct;54(5):439-451. doi: 10.1007/s10735-023-10153-6. Epub 2023 Sep 20. J Mol Histol. 2023. PMID: 37728670
-
Involvement of hypoxia-inducible factor-1α in the oxidative stress induced by advanced glycation end products in murine Leydig cells.Toxicol In Vitro. 2016 Apr;32:146-53. doi: 10.1016/j.tiv.2015.12.016. Epub 2015 Dec 30. Toxicol In Vitro. 2016. PMID: 26743761
-
Fine particulate matter (PM2.5) induces testosterone disruption by triggering ferroptosis through SIRT1/HIF-1α signaling pathway in male mice.Free Radic Biol Med. 2024 Aug 20;221:40-51. doi: 10.1016/j.freeradbiomed.2024.05.026. Epub 2024 May 15. Free Radic Biol Med. 2024. PMID: 38759901
-
Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics.Am J Kidney Dis. 2021 Feb;77(2):280-286. doi: 10.1053/j.ajkd.2020.04.016. Epub 2020 Jul 23. Am J Kidney Dis. 2021. PMID: 32711072 Review.
-
The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair.J Urol. 2004 Dec;172(6 Pt 2):2574-8. doi: 10.1097/01.ju.0000144203.30718.19. J Urol. 2004. PMID: 15538211 Review.
Cited by
-
Moxibustion prevents tripterygium glycoside-induced oligoasthenoteratozoospermia in rats via reduced oxidative stress and modulation of the Nrf2/HO-1 signaling pathway.Aging (Albany NY). 2024 Jan 25;16(3):2141-2160. doi: 10.18632/aging.205475. Epub 2024 Jan 25. Aging (Albany NY). 2024. PMID: 38277193 Free PMC article.
-
Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view.Reprod Biol Endocrinol. 2023 Sep 26;21(1):88. doi: 10.1186/s12958-023-01134-1. Reprod Biol Endocrinol. 2023. PMID: 37749573 Free PMC article. Review.
-
Obesity induced by a high-fat diet changes p62 protein levels in mouse reproductive organs.J Mol Histol. 2024 Nov 29;56(1):13. doi: 10.1007/s10735-024-10310-5. J Mol Histol. 2024. PMID: 39611975
-
Sphingolipids and Male Reproductive Health: A Narrative Review of Their Roles in Spermatogenesis, Fertility, and Dysfunction.Reprod Sci. 2025 Jul;32(7):2121-2136. doi: 10.1007/s43032-025-01898-4. Epub 2025 Jun 12. Reprod Sci. 2025. PMID: 40506656 Review.
-
Role of sphingolipid metabolites in the homeostasis of steroid hormones and the maintenance of testicular functions.Front Endocrinol (Lausanne). 2023 Mar 17;14:1170023. doi: 10.3389/fendo.2023.1170023. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008929 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

