Exosomes from Adipose-Derived Stem Cells Alleviate Dexamethasone-Induced Bone Loss by Regulating the Nrf2/HO-1 Axis
- PMID: 36778207
- PMCID: PMC9908349
- DOI: 10.1155/2023/3602962
Exosomes from Adipose-Derived Stem Cells Alleviate Dexamethasone-Induced Bone Loss by Regulating the Nrf2/HO-1 Axis
Abstract
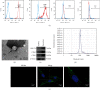
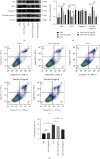
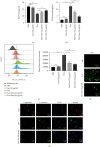
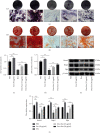
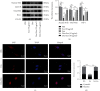
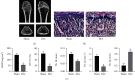
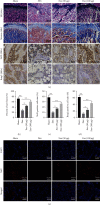
The widespread use of therapeutic glucocorticoids has increased the incidences of glucocorticoid-induced osteoporosis (GIOP). Oxidative stress and mitochondrial dysfunction are major causes of GIOP; therefore, alleviation of excess oxidative stress in osteoblasts is a potential therapeutic strategy for osteoporosis. Exosomes derived from ADSCs (ADSCs-Exos), as novel cell-free therapeutics, can modulate various biological processes, such as immunomodulation, reduce oxidative damage, and promote tissue repair as well as regeneration. In this study, ADSCs-Exos restored the viability and osteogenic potential of MC3T3-E1 cells by attenuating apoptosis, oxidative damage, intracellular ROS generation, and mitochondrial dysfunction. Moreover, after pretreatment with ADSCs-Exos, Nrf2 expressions were upregulated in Dex-stimulated osteoblasts. Inhibitory assays showed that silencing Nrf2 partially eliminated the protective effects of ADSCs-Exos. The rat model assays confirmed that ADSCs-Exos alleviated the Dex-induced increase in oxidation levels, restored bone mass of the distal femur, and increased the expressions of Nrf2 and osteogenic markers in bone tissues. Thus, ADSCs-Exos alleviated apoptosis and oxidative stress by regulating Nrf2/HO-1 expressions after Dex and prevented the development of GIOP in vivo.
Copyright © 2023 Xue-wei Yao et al.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

