Simvastatin Reduces NETosis to Attenuate Severe Asthma by Inhibiting PAD4 Expression
- PMID: 36778209
- PMCID: PMC9911252
- DOI: 10.1155/2023/1493684
Simvastatin Reduces NETosis to Attenuate Severe Asthma by Inhibiting PAD4 Expression
Abstract
Objective: Patients with severe asthma respond poorly to corticosteroids, and their care accounts for more than 60% of the total costs attributed to asthma. Neutrophils form neutrophil extracellular traps (NETs), which play a crucial role in severe asthma. Statins have shown anti-inflammatory effects by reducing NETosis. In this study, we investigate if simvastatin can attenuate severe asthma by reducing NETosis and the underlying mechanism.
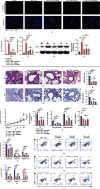
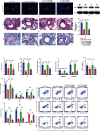
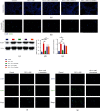
Methods: Mice were concomitantly sensitized with ovalbumin (OVA), house dust mite (HDM), and lipopolysaccharide (LPS) during sensitization to establish a mouse model of severe asthma with neutrophil predominant inflammation (OVA+LPS mice) and treated with or without simvastatin. In inflammatory response, proportions of Th2, Th17, and Treg cells in lung tissue were detected by flow cytometry, and the levels of cytokines, dsDNA, and MPO-DNA in bronchoalveolar lavage fluid (BALF) were analyzed by ELISA. Citrullinated histone H3 (CitH3) and peptidyl arginine deiminase 4 (PAD4) in lung tissue were determined by Western blot and immunofluorescence imaging. PAD4 mRNA was determined by quantitative PCR (qPCR). HL-60 cells were differentiated into neutrophil-like cells by 1.25% DMSO. The neutrophil-like cells were treated with or without LPS, and simvastatin was then stimulated with PMA. CitH3 and PAD4 expressions were determined.
Results: Sensitization with OVA, HDM, and LPS resulted in neutrophilic inflammation and the formation of NETs in the lungs. Simvastatin treatment reduced the inflammation score, cytokine levels, total cells, and neutrophil counts in the BALF and reduced proportions of Th2 and Th17 but increased Treg cells in lungs of OVA+LPS mice. Simvastatin-treated OVA+LPS mice show reduced NET formation in BALF and lung tissue compared to control mice. Adoptive transfer of neutrophils was sufficient to restore NETosis and neutrophilic inflammation in simvastatin-treated OVA+LPS mice. Simvastatin reduced PAD4 mRNA and protein expression in lung tissues and neutrophils isolated from lungs of OVA+LPS mice and consequent NET formation. In vitro, simvastatin reduced LPS-induced PAD4 upregulation and NETosis in HL-60-differentiated neutrophil-like cells. Furthermore, PAD4-overexpressed lentiviral transduction was sufficient to restore PAD4 protein expression and NETosis in simvastatin-treated HL-60-differentiated neutrophil-like cells.
Conclusions: Simvastatin reduces Th17-mediated neutrophilic inflammation and airway hyperreactivity by reducing PAD4 expression and inhibiting NETosis in a mouse model of severe asthma. Severe asthmatic patients with high levels of circulating NETs or sputum NETs may show improved responses to statin treatment.
Copyright © 2023 Yun-Rong Chen et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

