This is a preprint.
Minimal mRNA uptake and inflammatory response to COVID-19 mRNA vaccine exposure in human placental explants
- PMID: 36778281
- PMCID: PMC9915836
- DOI: 10.1101/2023.02.01.23285349
Minimal mRNA uptake and inflammatory response to COVID-19 mRNA vaccine exposure in human placental explants
Update in
-
Minimal mRNA uptake and inflammatory response to COVID-19 mRNA vaccine exposure in human placental explants.iScience. 2023 Aug 7;26(9):107549. doi: 10.1016/j.isci.2023.107549. eCollection 2023 Sep 15. iScience. 2023. PMID: 37664582 Free PMC article.
Abstract
Despite universal recommendations for COVID-19 mRNA vaccination in pregnancy, uptake has been lower than desired. There have been limited studies of the direct impact of COVID-19 mRNA vaccine exposure in human placental tissue. Using a primary human villous explant model, we investigated the uptake of two common mRNA vaccines (BNT162b2 Pfizer-BioNTech or mRNA-1273 Moderna), and whether exposure altered villous cytokine responses. Explants derived from second or third trimester chorionic villi were incubated with vaccines at supraphysiologic concentrations and analyzed at two time points. We observed minimal uptake of mRNA vaccines in placental explants by in situ hybridization and quantitative RT-PCR. No specific or global cytokine response was elicited by either of the mRNA vaccines in multiplexed immunoassays. Our results suggest that the human placenta does not readily absorb the COVID-19 mRNA vaccines nor generate a significant inflammatory response after exposure.
Figures

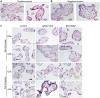
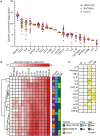
Similar articles
-
Minimal mRNA uptake and inflammatory response to COVID-19 mRNA vaccine exposure in human placental explants.iScience. 2023 Aug 7;26(9):107549. doi: 10.1016/j.isci.2023.107549. eCollection 2023 Sep 15. iScience. 2023. PMID: 37664582 Free PMC article.
-
[18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact.Eur Radiol. 2022 Jan;32(1):508-516. doi: 10.1007/s00330-021-08122-2. Epub 2021 Jun 22. Eur Radiol. 2022. PMID: 34156552 Free PMC article.
-
Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021.MMWR Morb Mortal Wkly Rep. 2021 Sep 24;70(38):1337-1343. doi: 10.15585/mmwr.mm7038e1. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34555004 Free PMC article.
-
Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review.BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
References
-
- Centers for Disease Control and Prevention. CDC COVID-19 Study Shows mRNA Vaccines Reduce Risk of Infection by 91 Percent for Fully Vaccinated People. https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html.
-
- Centers for Disease Control and Prevention. COVID-19 Vaccines While Pregnant or Breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregn.... - PubMed
-
- Centers for Disease Control and Prevention. COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
