This is a preprint.
Tubular Mitochondrial Pyruvate Carrier Disruption Elicits Redox Adaptations that Protect from Acute Kidney Injury
- PMID: 36778297
- PMCID: PMC9915694
- DOI: 10.1101/2023.01.31.526492
Tubular Mitochondrial Pyruvate Carrier Disruption Elicits Redox Adaptations that Protect from Acute Kidney Injury
Update in
-
Tubular mitochondrial pyruvate carrier disruption elicits redox adaptations that protect from acute kidney injury.Mol Metab. 2024 Jan;79:101849. doi: 10.1016/j.molmet.2023.101849. Epub 2023 Dec 7. Mol Metab. 2024. PMID: 38056691 Free PMC article.
Abstract
Energy-intensive kidney reabsorption processes essential for normal whole-body function are maintained by tubular epithelial cell metabolism. Tubular metabolism changes markedly following acute kidney injury (AKI), but which changes are adaptive versus maladaptive remain poorly understood. In publicly available data sets, we noticed a consistent downregulation of the mitochondrial pyruvate carrier (MPC) after AKI, which we experimentally confirmed. To test the functional consequences of MPC downregulation, we generated novel tubular epithelial cell-specific Mpc1 knockout (MPC TubKO) mice. 13C-glucose tracing, steady-state metabolomic profiling, and enzymatic activity assays revealed that MPC TubKO coordinately increased activities of the pentose phosphate pathway and the glutathione and thioredoxin oxidant defense systems. Following rhabdomyolysis-induced AKI, MPC TubKO decreased markers of kidney injury and oxidative damage and strikingly increased survival. Our findings suggest that decreased mitochondrial pyruvate uptake is a central adaptive response following AKI and raise the possibility of therapeutically modulating the MPC to attenuate AKI severity.
Keywords: acute kidney injury; metabolomics; mitochondrial metabolism; oxidative damage.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures
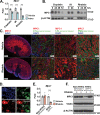
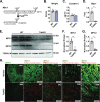

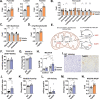



References
-
- Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–57. - PubMed
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, and Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. - PubMed
-
- Goldstein SL, Jaber BL, Faubel S, Chawla LS, and Acute Kidney Injury Advisory Group of American Society of N. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8(3):476–83. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
