This is a preprint.
IL-15 synergizes with CD40 agonist antibodies to induce durable immunity against bladder cancer
- PMID: 36778311
- PMCID: PMC9915460
- DOI: 10.1101/2023.01.30.526266
IL-15 synergizes with CD40 agonist antibodies to induce durable immunity against bladder cancer
Update in
-
IL-15 synergizes with CD40 agonist antibodies to induce durable immunity against bladder cancer.Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2306782120. doi: 10.1073/pnas.2306782120. Epub 2023 Aug 22. Proc Natl Acad Sci U S A. 2023. PMID: 37607227 Free PMC article.
Abstract
CD40 is a central co-stimulatory receptor implicated in the development of productive anti-tumor immune responses across multiple cancers, including bladder cancer. Despite strong preclinical rationale, systemic administration of therapeutic agonistic antibodies targeting the CD40 pathway have demonstrated dose limiting toxicities with minimal clinical activity to date, emphasizing an important need for optimized CD40-targeted approaches, including rational combination therapy strategies. Here, we describe an important role for the endogenous IL-15 pathway in contributing to the therapeutic activity of CD40 agonism in orthotopic bladder tumors, with upregulation of trans-presented IL-15/IL-15Rα surface complexes, particularly by cross-presenting cDC1s, and associated enrichment of activated CD8 T cells within the bladder tumor microenvironment. In bladder cancer patient samples, we identify DCs as the primary source of IL-15, however, they lack high levels of IL-15Rα at baseline. Using humanized immunocompetent orthotopic bladder tumor models, we demonstrate the ability to therapeutically augment this interaction through combined treatment with anti-CD40 agonist antibodies and exogenous IL-15, including the fully-human Fc-optimized antibody 2141-V11 currently in clinical development for the treatment of bladder cancer. Combination therapy enhances the crosstalk between Batf3-dependent cDC1s and CD8 T cells, driving robust primary anti-tumor activity and further stimulating long-term systemic anti-tumor memory responses associated with circulating memory-phenotype T and NK cell populations. Collectively, these data reveal an important role for IL-15 in mediating anti-tumor CD40 agonist responses in bladder cancer and provide key proof-of-concept for combined use of Fc-optimized anti-CD40 agonist antibodies and agents targeting the IL-15 pathway. These data support expansion of ongoing clinical studies evaluating anti-CD40 agonist antibodies and IL-15-based approaches to evaluate combinations of these promising therapeutics for the treatment of patients with bladder cancer.
Figures
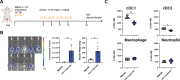




Similar articles
-
IL-15 synergizes with CD40 agonist antibodies to induce durable immunity against bladder cancer.Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2306782120. doi: 10.1073/pnas.2306782120. Epub 2023 Aug 22. Proc Natl Acad Sci U S A. 2023. PMID: 37607227 Free PMC article.
-
Dendritic cell targeting with Fc-enhanced CD40 antibody agonists induces durable antitumor immunity in humanized mouse models of bladder cancer.Sci Transl Med. 2021 May 19;13(594):eabd1346. doi: 10.1126/scitranslmed.abd1346. Sci Transl Med. 2021. PMID: 34011627 Free PMC article.
-
IL-15R alpha-IgG1-Fc enhances IL-2 and IL-15 anti-tumor action through NK and CD8+ T cells proliferation and activation.J Mol Cell Biol. 2010 Aug;2(4):217-22. doi: 10.1093/jmcb/mjq012. J Mol Cell Biol. 2010. PMID: 20671116
-
Agonistic CD40 Antibodies in Cancer Treatment.Cancers (Basel). 2021 Mar 15;13(6):1302. doi: 10.3390/cancers13061302. Cancers (Basel). 2021. PMID: 33804039 Free PMC article. Review.
-
IL-15 in the Combination Immunotherapy of Cancer.Front Immunol. 2020 May 19;11:868. doi: 10.3389/fimmu.2020.00868. eCollection 2020. Front Immunol. 2020. PMID: 32508818 Free PMC article. Review.
References
-
- Vonderheide R. H., CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med 71, 47–58 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
