This is a preprint.
Lung infection by P. aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice
- PMID: 36778380
- PMCID: PMC9915779
- DOI: 10.21203/rs.3.rs-2511441/v1
Lung infection by P. aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice
Update in
-
Lung infection by Pseudomonas aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice.J Neuroinflammation. 2023 May 27;20(1):127. doi: 10.1186/s12974-023-02817-7. J Neuroinflammation. 2023. PMID: 37245027 Free PMC article.
Abstract
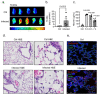
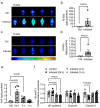
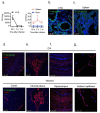
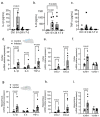
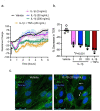
Background Severe lung infection can lead to brain dysfunction and neurobehavioral disorders. The mechanisms that regulate the lung-brain axis of inflammatory response to respiratory infection are incompletely understood. This study examined the effects of lung infection causing systemic and neuroinflammation as a potential mechanism contributing to blood-brain barrier (BBB) leakage and behavioral impairment. Methods Pneumonia was induced in adult C57BL/6 mice by intratracheal inoculation of Pseudomonas aeruginosa (PA). Solute extravasation, histology, immunofluorescence, RT-PCR, multiphoton imaging and neurological testing were performed in this study. Results Lung infection caused alveolar-capillary barrier injury as indicated by leakage of plasma proteins across pulmonary microvessels and histopathological characteristics of pulmonary edema (alveolar wall thickening, microvessel congestion, and neutrophil infiltration). PA also caused significant BBB dysfunction characterized by leakage of different sized molecules across cerebral microvessels and a decreased expression of cell-cell junctions (VE-cadherin, claudin-5) in the brain. BBB leakage peaked at 24 hours and lasted for 7 days post-inoculation. Additionally, mice with lung infection displayed hyperlocomotion and anxiety-like behaviors. To test whether cerebral dysfunction was caused by PA directly or indirectly, we measured bacterial load in multiple organs. While PA loads were detected in the lungs up to 7 days post-inoculation, bacteria were not detected in the brain as evidenced by negative cerebral spinal fluid (CSF) cultures and lack of distribution in different brain regions or isolated cerebral microvessels. However, mice with PA lung infection demonstrated increased mRNA expression in the brain of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), chemokines (CXCL-1, CXCL-2) and adhesion molecules (VCAM-1 and ICAM-1) along with CD11b + cell recruitment, corresponding to their increased blood levels of white cells (polymorphonuclear cells) and cytokines. To confirm the direct effect of cytokines on endothelial permeability, we measured cell-cell adhesive barrier resistance and junction morphology in mouse brain microvascular endothelial cell monolayers, where administration of IL-1β induced a significant reduction of barrier function coupled with tight junction (TJ) diffusion and disorganization. Combined treatment with IL-1β and TNFα augmented the barrier injury. Conclusions These results suggest that lung bacterial infection causes cerebral microvascular leakage and neuroinflammation via a mechanism involving cytokine-induced BBB injury.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests
Figures


References
-
- McManus RM, Higgins SC, Mills KH, Lynch MA. Respiratory infection promotes T cell infiltration and amyloid-beta deposition in APP/PS1 mice. Neurobiol Aging. 2014;35(1):109–21. - PubMed
-
- Rubin K, Glazer S. The pertussis hypothesis: Bordetella pertussis colonization in the pathogenesis of Alzheimer’s disease. Immunobiology. 2017;222(2):228–40. - PubMed
-
- Maheshwari P Eslick GD. Bacterial infection and Alzheimer’s disease: a meta-analysis. J Alzheimers Dis. 2015;43(3):957–66. - PubMed
-
- Janssens JP Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4(2):112–24. - PubMed
-
- Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–66. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

