This is a preprint.
DLK-dependent axonal mitochondrial fission drives degeneration following axotomy
- PMID: 36778383
- PMCID: PMC9915495
- DOI: 10.1101/2023.01.30.526132
DLK-dependent axonal mitochondrial fission drives degeneration following axotomy
Update in
-
DLK-dependent axonal mitochondrial fission drives degeneration after axotomy.Nat Commun. 2024 Dec 30;15(1):10806. doi: 10.1038/s41467-024-54982-9. Nat Commun. 2024. PMID: 39737939 Free PMC article.
Abstract
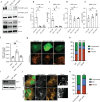
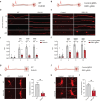
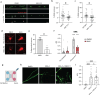
Currently there are no effective treatments for an array of neurodegenerative disorders to a large part because cell-based models fail to recapitulate disease. Here we developed a reproducible human iPSC-based model where laser axotomy causes retrograde axon degeneration leading to neuronal cell death. Time-lapse confocal imaging revealed that damage triggers an apoptotic wave of mitochondrial fission proceeding from the site of injury to the soma. We demonstrated that this apoptotic wave is locally initiated in the axon by dual leucine zipper kinase (DLK). We found that mitochondrial fission and resultant cell death are entirely dependent on phosphorylation of dynamin related protein 1 (DRP1) downstream of DLK, revealing a new mechanism by which DLK can drive apoptosis. Importantly, we show that CRISPR mediated Drp1 depletion protected mouse retinal ganglion neurons from degeneration after optic nerve crush. Our results provide a powerful platform for studying degeneration of human neurons, pinpoint key early events in damage related neural death and new focus for therapeutic intervention.
Figures




References
-
- Whitmore A.V., et al., The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ, 2003. 10(2): p. 260–1. - PubMed
-
- Deckwerth T.L., et al., BAX is required for neuronal death after trophic factor deprivation and during development. Neuron, 1996. 17(3): p. 401–11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
