This is a preprint.
Perturb-tracing enables high-content screening of multiscale 3D genome regulators
- PMID: 36778402
- PMCID: PMC9915657
- DOI: 10.1101/2023.01.31.525983
Perturb-tracing enables high-content screening of multiscale 3D genome regulators
Update in
-
Perturb-tracing enables high-content screening of multi-scale 3D genome regulators.Nat Methods. 2025 May;22(5):950-961. doi: 10.1038/s41592-025-02652-z. Epub 2025 Apr 10. Nat Methods. 2025. PMID: 40211002 Free PMC article.
Abstract
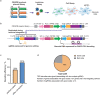
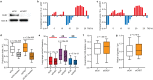
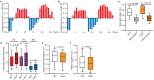
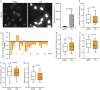
Three-dimensional (3D) genome organization becomes altered during development, aging, and disease1-23, but the factors regulating chromatin topology are incompletely understood and currently no technology can efficiently screen for new regulators of multiscale chromatin organization. Here, we developed an image-based high-content screening platform (Perturb-tracing) that combines pooled CRISPR screen, a new cellular barcode readout method (BARC-FISH), and chromatin tracing. We performed a loss-of-function screen in human cells, and visualized alterations to their genome organization from 13,000 imaging target-perturbation combinations, alongside perturbation-paired barcode readout in the same single cells. Using 1.4 million 3D positions along chromosome traces, we discovered tens of new regulators of chromatin folding at different length scales, ranging from chromatin domains and compartments to chromosome territory. A subset of the regulators exhibited 3D genome effects associated with loop-extrusion and A-B compartmentalization mechanisms, while others were largely unrelated to these known 3D genome mechanisms. We found that the ATP-dependent helicase CHD7, the loss of which causes the congenital neural crest syndrome CHARGE24 and a chromatin remodeler previously shown to promote local chromatin openness25-27, counter-intuitively compacts chromatin over long range in different genomic contexts and cell backgrounds including neural crest cells, and globally represses gene expression. The DNA compaction effect of CHD7 is independent of its chromatin remodeling activity and does not require other protein partners. Finally, we identified new regulators of nuclear architectures and found a functional link between chromatin compaction and nuclear shape. Altogether, our method enables scalable, high-content identification of chromatin and nuclear topology regulators that will stimulate new insights into the 3D genome functions, such as global gene and nuclear regulation, in health and disease.
Figures









References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
