This is a preprint.
Biochemical and Genetic Evidence Supports Fyv6 as a Second-Step Splicing Factor in Saccharomyces cerevisiae
- PMID: 36778415
- PMCID: PMC9915624
- DOI: 10.1101/2023.01.30.526368
Biochemical and Genetic Evidence Supports Fyv6 as a Second-Step Splicing Factor in Saccharomyces cerevisiae
Update in
-
Biochemical and genetic evidence supports Fyv6 as a second-step splicing factor in Saccharomyces cerevisiae.RNA. 2023 Nov;29(11):1792-1802. doi: 10.1261/rna.079607.123. Epub 2023 Aug 25. RNA. 2023. PMID: 37625852 Free PMC article.
Abstract
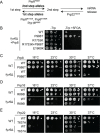
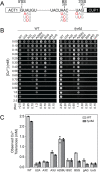
Precursor mRNA (pre-mRNA) splicing is an essential process for gene expression in eukaryotes catalyzed by the spliceosome in two transesterification steps. The spliceosome is a large, highly dynamic complex composed of 5 small nuclear RNAs and dozens of proteins, some of which are needed throughout the splicing reaction while others only act during specific stages. The human protein FAM192A was recently proposed to be a splicing factor that functions during the second transesterification step, exon ligation, based on analysis of cryo-electron microscopy (cryo-EM) density. It was also proposed that Fyv6 might be the functional S. cerevisiae homolog of FAM192A; however, no biochemical or genetic data has been reported to support this hypothesis. Herein, we show that Fyv6 is a splicing factor and acts during exon ligation. Deletion of FYV6 results in genetic interactions with the essential splicing factors Prp8, Prp16, and Prp22; decreases splicing in vivo of reporter genes harboring intron substitutions that limit the rate of exon ligation; and changes 3’ splice site (SS) selection. Together, these data suggest that Fyv6 is a component of the spliceosome and the potential functional and structural homolog of human FAM192A.
Conflict of interest statement
COMPETING INTERESTS
AAH is a member of the scientific advisory board and carrying out sponsored research for Remix Therapeutics.
Figures


References
-
- Bastian M, Heymann S, Jacomy M. 2009. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proceedings of the International AAAI Conference on Web and Social Media 3, 361–362. doi:10.1609/icwsm.v3i1.13937 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
