This is a preprint.
Peripheral immune-derived matrix metalloproteinase promotes stress susceptibility
- PMID: 36778505
- PMCID: PMC9915787
- DOI: 10.21203/rs.3.rs-1647827/v1
Peripheral immune-derived matrix metalloproteinase promotes stress susceptibility
Update in
-
Circulating myeloid-derived MMP8 in stress susceptibility and depression.Nature. 2024 Feb;626(8001):1108-1115. doi: 10.1038/s41586-023-07015-2. Epub 2024 Feb 7. Nature. 2024. PMID: 38326622 Free PMC article.
Abstract
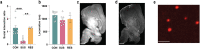
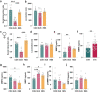
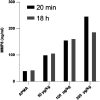
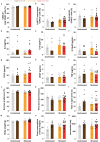
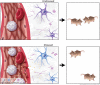
Psychosocial stress has profound effects on the body, including the peripheral immune system and the brain1,2. Although a large number of pre-clinical and clinical studies have linked peripheral immune system alterations to stress-related disorders such as major depressive disorder (MDD)3,4,5, the underlying mechanisms are not well understood. Here we show that a peripheral myeloid cell-specific proteinase, matrix metalloproteinase 8 (MMP8), is elevated in serum of subjects with MDD as well as in stress-susceptible (SUS) mice following chronic social defeat stress (CSDS). In mice, we show that this increase leads to alterations in extracellular space and neurophysiological changes in the nucleus accumbens (NAc), thereby altering social behaviour. Using a combination of mass cytometry and single-cell RNA-sequencing, we performed high-dimensional phenotyping of immune cells in circulation and brain and demonstrate that peripheral monocytes are strongly affected by stress. Both peripheral and brain-infiltrating monocytes of SUS mice showed increased Mmp8 expression following CSDS. We further demonstrate that peripheral MMP8 directly infiltrates the NAc parenchyma to control the ultrastructure of the extracellular space. Depleting MMP8 prevented stress-induced social avoidance behaviour and alterations in NAc neurophysiology and extracellular space. Collectively, these data establish a novel mechanism by which peripheral immune factors can affect central nervous system function and behaviour in the context of stress. Targeting specific peripheral immune cell-derived matrix metalloproteinases could constitute novel therapeutic targets for stress-related neuropsychiatric disorders.
Figures

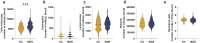

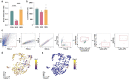

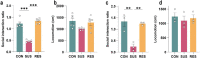





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

