Factors Associated With Fecal Calprotectin Sample Collection Compliance: An IBD Center Quality Improvement Project
- PMID: 36778515
- PMCID: PMC9802166
- DOI: 10.1093/crocol/otac042
Factors Associated With Fecal Calprotectin Sample Collection Compliance: An IBD Center Quality Improvement Project
Abstract
Background: Fecal calprotectin (Fcal) is a noninvasive, inexpensive biomarker of disease activity. However, patient compliance with this test is variable and incompletely described. We assessed compliance rates with Fcal tests and identified factors associated with noncompliance.
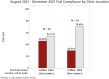
Methods: A retrospective chart review of patients with inflammatory bowel disease (IBD) who had a Fcal test ordered through our center between August 2021 and December 2021 was conducted. Demographic, clinical, disease, and test-related information were recorded. Patients with incomplete Fcal orders were sent a survey to better understand their reasons for noncompliance. Simple statistical analysis and and multivariable logistic regression modeling were performed.
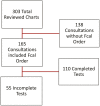
Results: Of 303 patients, 165 (54.4%) had an order for Fcal. Of the Fcal tests ordered, 55 (33.3%) were not completed. Remission of IBD, no prior Fcal completion, and tests ordered at a distant site were all associated with test noncompletion. A multivariable logistic regression revealed that history of a prior completed Fcal test is associated with subsequent test completion (odds ratio = 2.1, 95% confidence interval 1.9-35.5, P = .004). Patients who did not complete the test described the pandemic and third-party testing center issues as the most common reasons for noncompliance.
Conclusions: In this single center experience with Fcal testing in patients with IBD, we identified that a history of incomplete Fcal testing and distant location of lab testing were significantly associated with noncompletion of the test. We provide practical guidance for future utilization and compliance, including the impact of home-based testing.
Keywords: IBD management; compliance; fecal calprotectin; noninvasive; test completion.
© The Author(s) 2022. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Figures
Similar articles
-
Impact of Fecal Calprotectin Measurement on Decision-making in Children with Inflammatory Bowel Disease.Front Pediatr. 2017 Jan 25;5:7. doi: 10.3389/fped.2017.00007. eCollection 2017. Front Pediatr. 2017. PMID: 28180127 Free PMC article.
-
Validation of the newly FDA-approved Buhlmann fCal Turbo assay for measurement of fecal calprotectin in a pediatric population.Pract Lab Med. 2020 Oct 17;22:e00178. doi: 10.1016/j.plabm.2020.e00178. eCollection 2020 Nov. Pract Lab Med. 2020. PMID: 33134465 Free PMC article.
-
Diagnostic Accuracy of Fecal Calprotectin for Pediatric Inflammatory Bowel Disease in Primary Care: A Prospective Cohort Study.Ann Fam Med. 2016 Sep;14(5):437-45. doi: 10.1370/afm.1949. Ann Fam Med. 2016. PMID: 27621160 Free PMC article.
-
Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin?Intest Res. 2016 Jan;14(1):5-14. doi: 10.5217/ir.2016.14.1.5. Epub 2016 Jan 25. Intest Res. 2016. PMID: 26884729 Free PMC article. Review.
-
Faecal calprotectin in inflammatory bowel diseases: a review focused on meta-analyses and routine usage limitations.Clin Chem Lab Med. 2019 Aug 27;57(9):1295-1307. doi: 10.1515/cclm-2018-1063. Clin Chem Lab Med. 2019. PMID: 30785706 Review.
Cited by
-
Remote Monitoring for Patients With Inflammatory Bowel Disease: Current Status and the Future of the Field.Gastroenterol Hepatol (N Y). 2024 Oct;20(10):621-627. Gastroenterol Hepatol (N Y). 2024. PMID: 40191010 Free PMC article.
-
Development of Luminescent Biosensors for Calprotectin.ACS Chem Biol. 2024 Jun 21;19(6):1250-1259. doi: 10.1021/acschembio.4c00265. Epub 2024 Jun 6. ACS Chem Biol. 2024. PMID: 38843544 Free PMC article.
References
-
- Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ.. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–297. - PubMed
-
- Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF.. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18(7):1356–1363. - PubMed
-
- Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis—PubMed. Accessed June 10, 2022. https://pubmed.ncbi.nlm.nih.gov/14762782/ - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous