Effect of a modified regimen on drug-sensitive retreated pulmonary tuberculosis: A multicenter study in China
- PMID: 36778546
- PMCID: PMC9909400
- DOI: 10.3389/fpubh.2023.1039399
Effect of a modified regimen on drug-sensitive retreated pulmonary tuberculosis: A multicenter study in China
Abstract
Background and objective: Retreatment pulmonary tuberculosis (PTB) still accounts for a large proportion of tuberculosis, and the treatment outcome is unfavorable. The recurrence of retreatment PTB based on long-term follow-up has not been well demonstrated. This study aimed to evaluate effect of a modified regimen on drug-sensitive retreated pulmonary tuberculosis.
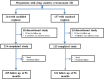
Methods: This multicenter cohort study was conducted in 29 hospitals from 23 regions of China from July 1, 2009, to December 31, 2020. Patients were divided into two treatment regimen groups including experimental group [modified regimen (4H-Rt2-E-Z-S(Lfx)/4H-Rt2-E)]and control group [standard regimen (2H-R-E-Z-S/6H-R-E or 3H-R-E-Z/6H-R-E)]. The patients enrolled were followed up of 56 months after successful treatment. We compared the treatment success rate, treatment failure rate, adverse reaction rate, and recurrence rate between two regimens. Multivariate Cox regression model was used to identify the potential risk factors for recurrence after successful treatment with proportional hazards assumptions tested for all variables.
Results: A total of 381 patients with retreatment PTB were enrolled, including 244 (64.0%) in the experimental group and 137 (36.0%) in the control group. Overall, the treatment success rate was significant higher in the experimental group than control group (84.0 vs. 74.5%, P = 0.024); no difference was observed in adverse reactions between the two groups (25.8 vs. 21.2%, P > 0.05). A total of 307 patients completed the 56 months of follow-up, including 205 with the modified regimen and 102 with the standard regimen. Among these, 10 cases (3.3%) relapsed, including 3 in the experimental group and 7 in the control group (1.5% vs 6.9%, P = 0.035). Reduced risks of recurrence were observed in patients treated with the modified regimen compared with the standard regimen, and the adjusted hazard ratio was 0.19 (0.04-0.77).
Conclusion: The modified retreatment regimen had more favorable treatment effects, including higher treatment success rate and lower recurrence rate in patients with retreated drug-sensitive PTB.
Keywords: follow-up; modified regimen; pulmonary tuberculosis; recurrence; retreatment.
Copyright © 2023 Ge, Ma, Zhang, Ma, Zhao, Chen, Huang, Shu, Chen, Wang, Li, Han, Shi, Wang, Li, Yang, Cao, Liu, Chen, Wu, Ouyang, Wang, Li, Wu, Xi, Leng, Zhang, Li, Li, Yang, Zhang, Cui, Liu, Kong, Sun, Du and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Improvement cues of lesion absorption using the adjuvant therapy of traditional Chinese medicine Qinbudan tablet for retreatment pulmonary tuberculosis with standard anti-tuberculosis regimen.Infect Dis Poverty. 2020 May 7;9(1):50. doi: 10.1186/s40249-020-00660-z. Infect Dis Poverty. 2020. PMID: 32381098 Free PMC article. Clinical Trial.
-
[The effectiveness of individualized treatment regimen on smear-positive retreatment pulmonary tuberculosis with mono- and poly-drug resistance].Zhonghua Jie He He Hu Xi Za Zhi. 2018 Jan 12;41(1):25-31. doi: 10.3760/cma.j.issn.1001-0939.2018.01.008. Zhonghua Jie He He Hu Xi Za Zhi. 2018. PMID: 29343012 Clinical Trial. Chinese.
-
Risk factors for the treatment outcome of retreated pulmonary tuberculosis patients in China: an optimized prediction model.Epidemiol Infect. 2017 Jul;145(9):1805-1814. doi: 10.1017/S0950268817000656. Epub 2017 Apr 11. Epidemiol Infect. 2017. PMID: 28397611 Free PMC article.
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
-
[Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients].Kekkaku. 2001 Jan;76(1):33-43. Kekkaku. 2001. PMID: 11211781 Review. Japanese.
Cited by
-
Update in tuberculosis treatment: a scoping review of current practices.Breathe (Sheff). 2025 Mar 18;21(1):240232. doi: 10.1183/20734735.0232-2024. eCollection 2025 Jan. Breathe (Sheff). 2025. PMID: 40104253 Free PMC article. Review.
References
-
- World Health Organization . Global tuberculosis report 2020. Geneva: World Health Organization; (2020).
-
- Yoshiyama T, Shrestha B, Maharjan B. Risk of relapse and failure after retreatment with the Category II regimen in Nepal. Int J Tuberc Lung Dis. (2010) 14:1418–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

