Evaluation of anakinra in the management of patients with COVID-19 infection: A randomized clinical trial
- PMID: 36778864
- PMCID: PMC9910697
- DOI: 10.3389/fmicb.2023.1098703
Evaluation of anakinra in the management of patients with COVID-19 infection: A randomized clinical trial
Abstract
Background: The global COVID-19 pandemic led to substantial clinical and economic outcomes with catastrophic consequences. While the majority of cases has mild to moderate disease, minority of patients progress into severe disease secondary to the stimulation of the immune response. The hyperinflammatory state contributes towards progression into multi-organ failure which necessitates suppressive therapy with variable outcomes. This study aims to explore the safety and efficacy of anakinra in COVID-19 patients with severe disease leading to cytokine release syndromes.
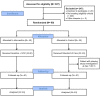
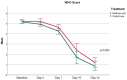
Methods: In this open-label, multi-center, randomized clinical trial, patients with confirmed COVID-19 infection with evidence of respiratory distress and signs of cytokine release syndrome were randomized in 1:1 ratio to receive either standard of care (SOC) or anakinra (100 mg subcutaneously every 12 h for 3 days then 100 mg subcutaneously once daily for 4 days) in addition to SOC. The primary outcome was treatment success at day 14 as defined by the WHO clinical progression score of ≤3. Primary analysis was based upon intention-to-treat population, with value of p of <0.05.
Results: Out 327 patients screened for eligibility, 80 patients were recruited for the study. The mean age was 49.9 years (SD = 11.7), with male predominance at 82.5% (n = 66). The primary outcome was not statistically different (87.5% (n = 35) in anakinra group vs. 92.5% (n = 37) in SOC group, p = 0.712; OR = 1.762 (95%CI: 0.39-7.93). The majority of reported adverse events were mild in severity and not related to the study treatment. Elevated aspartate aminotransferase was the only significant adverse event which was not associated with discontinuation of therapy.
Conclusion: In patients with severe COVID-19 infection, the addition of anakinra to SOC treatment was safe but was not associated with significant improvement according to the WHO clinical progression scale. Further studies are warranted to explore patients' subgroups characteristics that might benefit from administered therapy.
Clinical trial registration: Trial registration at ClinicalTrials.gov, identifier: NCT04643678.
Keywords: COVID-19; SARS-CoV-2; anakinra; cytokine release syndrome; interlukin-1 inhibitor; pneumonia.
Copyright © 2023 Elmekaty, Maklad, Abouelhassan, Munir, Ibrahim, Nair, Alibrahim, Iqbal, Al Bishawi, Abdelmajid, Aboukamar, Hadi, Khattab, Al Soub and Al Maslamani.
Conflict of interest statement
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):468. doi: 10.1186/s13063-020-04453-5. Trials. 2020. PMID: 32493441 Free PMC article.
-
Efficacy and Safety of Anakinra Plus Standard of Care for Patients With Severe COVID-19: A Randomized Phase 2/3 Clinical Trial.JAMA Netw Open. 2023 Apr 3;6(4):e237243. doi: 10.1001/jamanetworkopen.2023.7243. JAMA Netw Open. 2023. PMID: 37027155 Free PMC article. Clinical Trial.
-
Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19).Trials. 2021 Jan 4;22(1):1. doi: 10.1186/s13063-020-04976-x. Trials. 2021. PMID: 33397449 Free PMC article.
-
From Cytokine Storm to Cytokine Breeze: Did Lessons Learned from Immunopathogenesis Improve Immunomodulatory Treatment of Moderate-to-Severe COVID-19?Biomedicines. 2022 Oct 18;10(10):2620. doi: 10.3390/biomedicines10102620. Biomedicines. 2022. PMID: 36289881 Free PMC article. Review.
Cited by
-
An Update on SARS-CoV-2 Clinical Trial Results-What We Can Learn for the Next Pandemic.Int J Mol Sci. 2023 Dec 26;25(1):354. doi: 10.3390/ijms25010354. Int J Mol Sci. 2023. PMID: 38203525 Free PMC article. Review.
-
IL-1 receptor antagonist: etiological and drug delivery systems overview.Inflamm Res. 2024 Dec;73(12):2231-2247. doi: 10.1007/s00011-024-01960-y. Epub 2024 Oct 26. Inflamm Res. 2024. PMID: 39455436 Review.
-
Efficacy of additional hemoperfusion in hospitalized patients with severe to critical COVID-19 disease.Sci Rep. 2024 Jul 31;14(1):17651. doi: 10.1038/s41598-024-68592-4. Sci Rep. 2024. PMID: 39085334 Free PMC article.
-
Innate immunity, therapeutic targets and monoclonal antibodies in SARS-CoV-2 infection.PeerJ. 2025 Jun 20;13:e19462. doi: 10.7717/peerj.19462. eCollection 2025. PeerJ. 2025. PMID: 40552037 Free PMC article. Review.
References
-
- Audemard-Verger A., Le Gouge A., Pestre V., Courjon J., Langlois V., Vareil M. O., et al. . (2022). Efficacy and safety of anakinra in adults presenting deteriorating respiratory symptoms from COVID-19: a randomized controlled trial. PLoS One 17:e0269065. doi: 10.1371/journal.pone.0269065, PMID: - DOI - PMC - PubMed
-
- Balkhair A., Al-Zakwani I., Al Busaidi M., Al-Khirbash A., Al Mubaihsi S., BaTaher H., et al. . (2021). Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int. J. Infect. Dis. 103, 288–296. doi: 10.1016/j.ijid.2020.11.149, PMID: - DOI - PMC - PubMed
-
- Barkas F., Filippas-Ntekouan S., Kosmidou M., Liberopoulos E., Liontos A., Milionis H. (2021). Anakinra in hospitalized non-intubated patients with coronavirus disease 2019: A systematic review and meta-analysis. Rheumatology 60, 5527–5537. doi: 10.1093/rheumatology/keab447, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous