Group A streptococci induce stronger M protein-fibronectin interaction when specific human antibodies are bound
- PMID: 36778879
- PMCID: PMC9909010
- DOI: 10.3389/fmicb.2023.1069789
Group A streptococci induce stronger M protein-fibronectin interaction when specific human antibodies are bound
Abstract
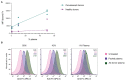
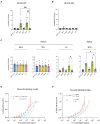
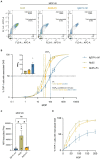
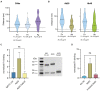
Group A streptococcus (GAS) is a highly adapted, human-specific pathogen that is known to manipulate the immune system through various mechanisms. GAS' M protein constitutes a primary target of the immune system due to its spatial configuration and dominance on the bacterial surface. Antibody responses targeting the M protein have been shown to favor the conserved C region. Such antibodies (Abs) circumvent antigenic escape and efficiently bind to various M types. The ability of GAS to bind to fibronectin (Fn), a high molecular weight glycoprotein of the extracellular matrix, has long been known to be essential for the pathogen's evolutionary success and fitness. However, some strains lack the ability to efficiently bind Fn. Instead, they have been found to additionally bind Fn via the A-B domains of their M proteins. Here, we show that human Abs can induce increased Fn-binding affinity in M proteins, likely by enhancing the weak A-B domain binding. We found that this enhanced Fn binding leads to a reduction in Ab-mediated phagocytosis, indicating that this constitutes a GAS immune escape mechanism. We could show that the Fc domain of Abs is necessary to trigger this phenomenon and that Ab flexibility may also play a key role. We, moreover, saw that our Abs could enhance Fn binding in 3 out of 5 emm type strains tested, belonging to different clades, making it likely that this is a more generalizable phenomenon. Together our results suggest a novel synergistic interplay of GAS and host proteins which ultimately benefits the bacterium.
Keywords: adaptive immunity; antibodies; co-evolution; fibronectin; group A – beta hemolytic streptococcus; immune subversion.
Copyright © 2023 Wrighton, Ahnlide, André, Bahnan and Nordenfelt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Integrin α5β1, as a Receptor of Fibronectin, Binds the FbaA Protein of Group A Streptococcus To Initiate Autophagy during Infection.mBio. 2020 Jun 9;11(3):e00771-20. doi: 10.1128/mBio.00771-20. mBio. 2020. PMID: 32518187 Free PMC article.
-
A Giant Extracellular Matrix Binding Protein of Staphylococcus epidermidis Binds Surface-Immobilized Fibronectin via a Novel Mechanism.mBio. 2020 Oct 20;11(5):e01612-20. doi: 10.1128/mBio.01612-20. mBio. 2020. PMID: 33082256 Free PMC article.
-
Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains.Mol Microbiol. 1996 Jul;21(2):373-84. doi: 10.1046/j.1365-2958.1996.6331356.x. Mol Microbiol. 1996. PMID: 8858591
-
Pleiotropic virulence factor - Streptococcus pyogenes fibronectin-binding proteins.Cell Microbiol. 2013 Apr;15(4):503-11. doi: 10.1111/cmi.12083. Epub 2012 Dec 20. Cell Microbiol. 2013. PMID: 23190012 Review.
-
Peritonsillar abscess: clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess.Dan Med J. 2017 Mar;64(3):B5333. Dan Med J. 2017. PMID: 28260599 Review.
Cited by
-
Streptococcus pyogenes: Pathogenesis and the Current Status of Vaccines.Vaccines (Basel). 2023 Sep 21;11(9):1510. doi: 10.3390/vaccines11091510. Vaccines (Basel). 2023. PMID: 37766186 Free PMC article. Review.
-
The hinge-engineered IgG1-IgG3 hybrid subclass IgGh47 potently enhances Fc-mediated function of anti-streptococcal and SARS-CoV-2 antibodies.Nat Commun. 2024 Apr 27;15(1):3600. doi: 10.1038/s41467-024-47928-8. Nat Commun. 2024. PMID: 38678029 Free PMC article.
-
Ocular Tics and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS).Diseases. 2024 Apr 25;12(5):83. doi: 10.3390/diseases12050083. Diseases. 2024. PMID: 38785738 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

