Effect of β - hydroxy - γ -aminophosphonate (β - HPC) on the hydrolytic activity of Nocardia brasiliensis as determined by FT-IR spectrometry
- PMID: 36778890
- PMCID: PMC9909415
- DOI: 10.3389/fmicb.2023.1089156
Effect of β - hydroxy - γ -aminophosphonate (β - HPC) on the hydrolytic activity of Nocardia brasiliensis as determined by FT-IR spectrometry
Abstract
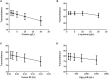
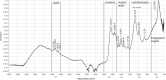
The use of immunomodulatory and metabolic modulating drugs has been considered a better strategy to improve the efficacy of conventional treatments against pathogens and metabolic diseases. L-carnitine is relevant in fatty acid metabolism and energy production by β-oxidation, but it also has a beneficial therapeutic immunomodulatory effect. The β-hydroxy-γ-aminophosphonate (β-HPC) was developed, synthesized and studied in different pathologies as a more soluble and stable analog than L-carnitine, which has been studied in bacterial physiology and metabolism; therefore, we set out to investigate the direct effect of β-HPC on the metabolism of N. brasiliensis, which causes actinomycetoma in Mexico and is underdiagnosed. To analyze the effect of β-HPC on the metabolic capacity of the bacterium for the hydrolysis of substrate casein, L-tyrosine, egg yolk, and tween 80, Fourier transform infrared spectroscopy (FT-IR) was employed. It was found that β-HPC increases the metabolic activity of N. brasiliensis associated with increased growth and increased hydrolysis of the substrates tested. By the effect of β-HPC, it was observed that, in the hydrolysis of L-tyrosine, the aromatic ring and functional groups were degraded. At 1515 cm-1, any distinctive signal or peak for this amino acid was missing, almost disappearing at 839, 720, 647, and 550 cm-1. In casein, hydrolysis is enhanced in the substrate, which is evident by the presence of NH, OH, amide, and CO. In casein, hydrolysis is enhanced in the substrate, which is evident by the presence of NH, OH, amide, COO, and P = O signals, characteristic of amino acids, in addition to the increase of the amide I and II bands. In Tween 80 the H-C = and C = C signals disappear and the ether signals are concentrated, it was distinguished by the intense band at 1100 cm-1. Egg yolk showed a large accumulation of phosphate groups at 1071 cm-1, where phosvitin is located. FT-IR has served to demonstrate that β-HPC is a hydrolysis enhancer. Furthermore, by obtaining the spectrum of N. brasiliensis, we intend to use it as a quick comparison tool with other spectra related to actinobacteria. Eventually, FT-IR may serve as a species identification option.
Keywords: FT-IR; L-carnitine analog; L-tyrosine; Nocardia brasiliensis spectrum; Tween 80; casein; egg yolk; hydrolysis.
Copyright © 2023 Martínez-Robles, González-Ballesteros, Reyes-Esparza, Trejo-Teniente, Jaramillo-Loranca, Téllez-Jurado, Vázquez-Valadez, Angeles and Vargas Hernández.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer FH declared a shared affiliation with several of the authors, EG-B, VV-V, EA, and SM-R, to the handling editor at the time of review.
Figures










Similar articles
-
Real time observation of proteolysis with Fourier transform infrared (FT-IR) and UV-circular dichroism spectroscopy: watching a protease eat a protein.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Jun;79(1):104-11. doi: 10.1016/j.saa.2011.01.055. Epub 2011 Feb 18. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21398173
-
Nocardia brasiliensis cell wall lipids modulate macrophage and dendritic responses that favor development of experimental actinomycetoma in BALB/c mice.Infect Immun. 2012 Oct;80(10):3587-601. doi: 10.1128/IAI.00446-12. Epub 2012 Jul 30. Infect Immun. 2012. PMID: 22851755 Free PMC article.
-
Structural analysis of some soluble elastins by means of FT-IR and 2D IR correlation spectroscopy.Biopolymers. 2010 Dec;93(12):1072-84. doi: 10.1002/bip.21524. Biopolymers. 2010. PMID: 20665685
-
Vibrational characterization of L-leucine phosphonate analogues: FT-IR, FT-Raman, and SERS spectroscopy studies and DFT calculations.J Phys Chem A. 2011 Oct 13;115(40):11067-78. doi: 10.1021/jp207101m. Epub 2011 Sep 19. J Phys Chem A. 2011. PMID: 21888349
-
FT-IR study of pyridoxamine 5' phosphate.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):83-7. doi: 10.1016/s1570-9639(03)00061-x. Biochim Biophys Acta. 2003. PMID: 12686113 Review.
References
-
- Aguado-Santacruz G. A., Moreno-Gómez M., Jiménez-Francisco B., García-Moya E., Preciado-Ortiz R. E. (2012). Impacto de los sideróforos microbianos y fitsideróforos en la asimilación de hierro por las plantas: Una síntesis. Rev. Fitotec. Mex. 35 9–21. 10.35196/rfm.2012.1.9 - DOI
-
- Amiel C., Curk M. C., Denis J., Ingouf J. C., Reyrolle J., Travert J. (1997). “Identification and classification of bacteria by fourier transform infrared spectroscopy (FTIR),” in En Spectroscopy of Biological Molecules: Modern Trends, eds Carmona P., Navarro P., Hernanz A. (Dordrecht: Springer Netherlands; ), 435–436. 10.1007/978-94-011-5622-6_194 - DOI
-
- Asai T., Shyuko K., Yasumasa K. (1963). Infrared absorption spectra of whole cells of nocardia and related organisms. J. Gen. App. Microbiol. 9 119–136. 10.2323/jgam.9.119 - DOI
-
- Astorga J. M. (2014). Aplicación de modelos de regresión lineal para determinar las armónicas de tensión y corriente. Ing. Energética 35 234–241.
LinkOut - more resources
Full Text Sources

