Synthesis and biological evaluation of coumarin-quinone hybrids as multifunctional bioactive agents
- PMID: 36778907
- PMCID: PMC9909729
- DOI: 10.5599/admet.1468
Synthesis and biological evaluation of coumarin-quinone hybrids as multifunctional bioactive agents
Abstract
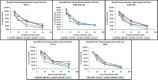
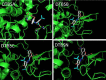
We report the synthesis, structural characterization and pharmaceutical activity of four coumarin-quinone hybrids. The compounds were significantly active against Staphylococcus aureus, Pseudomonas aeoginosa and Candida albicans. Promising antioxidant activity was observed when compared to ascorbic acid. Two compounds, DTBSB and DTBSN, also showed commendable in vitro antiproliferative activities against the cells of human cancer cell lines MCF-7, MDA-MB-231, COLO-205, HT-29 and A549 along with appreciable tumor selectivity with distinct selectivity index. Molecular docking studies using cyclooxygenase-2 (PDB ID: 6COX) revealed strong binding affinities for the COX-2 active site. Moreover, ADMET properties of the synthesized compounds were determined using the pKCSM and SwissADME online tools and all the compounds had accurate pharmacokinetic profiles. Hence, the new coumarin-quinone hybrids DTBSB and DTBSN can be considered for optimization and lead development.
Keywords: ADMET; Antimicrobial; Antioxidant; Antiproliferative; Molecular Docking; drug-likeness.
Copyright © 2022 by the authors.
Conflict of interest statement
Conflict of interest: The conflict of interest does not exist.
Figures



Similar articles
-
One-Pot Synthesis of Novel Hydrazono-1,3-Thıazolıdın-4-One Derivatives as Anti-HIV and Anti-Tubercular Agents: Synthesıs, Bıologıcal Evaluatıon, Molecular Modelling and Admet Studıes.Curr HIV Res. 2022;20(3):255-271. doi: 10.2174/1570162X20666220512163049. Curr HIV Res. 2022. PMID: 35549861
-
Betulin-1,4-quinone hybrids: Synthesis, anticancer activity and molecular docking study with NQO1 enzyme.Eur J Med Chem. 2019 Sep 1;177:302-315. doi: 10.1016/j.ejmech.2019.05.063. Epub 2019 May 24. Eur J Med Chem. 2019. PMID: 31158746
-
Chemical Characterization, In-silico Evaluation, and Molecular Docking Analysis of Antiproliferative Compounds Isolated from the Bark of Anthocephalus cadamba Miq.Anticancer Agents Med Chem. 2022;22(20):3416-3437. doi: 10.2174/1871520622666220204123348. Anticancer Agents Med Chem. 2022. PMID: 35125087
-
Synthesis, Cytotoxicity and Antimicrobial Evaluation of New Coumarin-Tagged β-Lactam Triazole Hybrid.Chem Biodivers. 2020 Jan;17(1):e1900462. doi: 10.1002/cbdv.201900462. Epub 2020 Jan 9. Chem Biodivers. 2020. PMID: 31788939
-
Hybrids of Coumarin Derivatives as Potent and Multifunctional Bioactive Agents: A Review.Med Chem. 2020;16(3):272-306. doi: 10.2174/1573406415666190416121448. Med Chem. 2020. PMID: 31038071 Review.
Cited by
-
Identification of DprE1 inhibitors for tuberculosis through integrated in-silico approaches.Sci Rep. 2024 May 17;14(1):11315. doi: 10.1038/s41598-024-61901-x. Sci Rep. 2024. PMID: 38760437 Free PMC article.
References
-
- Nofal Z.M., El-Zahar M. I., Abd El-Karim S.S. Novel coumarin derivatives with expected biological activity. Molecules 5(2) (2000) 99-113. https://doi.org/10.3390/50200099. 10.3390/50200099 - DOI
-
- Pangal A., Mujahid Y., Desai B., Shaikh J.A., Ahmed K. Synthesis of 3-(2-(subsituted-(trifluoromethyl)phenylamino)acetyl)-2H-chromen-2-one derivatives as new anticancer agents. Current Chemistry Letters 11(1) (2022) 105–112. https://doi.org/10.5267/j.ccl.2021.8.004. 10.5267/j.ccl.2021.8.004 - DOI
-
- Wu Y., Xu J., Liu Y., Zeng Y., Wu G. A review on antitumor mechanisms of coumarins. Front. Oncol. 10 (2020) 592853. https://doi.org/10.3389/fonc.2020.592853. 10.3389/fonc.2020.592853 - DOI - PMC - PubMed
-
- Song X.F., Fan J., Liu L., Liu X.F., Gao F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. (Weinheim) 353 (2020) e2000025. https://doi.org/10.1002/ardp.202000025 10.1002/ardp.202000025 - DOI - PubMed
-
- Kontogiorgis C., Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J Enzyme Inhib. Med. Chem. 18 (2003) 63-69. https://doi.org/10.1080/1475636031000069291 10.1080/1475636031000069291 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
