Evaluation of pooling strategy of SARS-CoV-2 RT-PCR in limited resources setting in Egypt at low prevalence
- PMID: 36778967
- PMCID: PMC9906572
- DOI: 10.1007/s00580-023-03445-6
Evaluation of pooling strategy of SARS-CoV-2 RT-PCR in limited resources setting in Egypt at low prevalence
Abstract
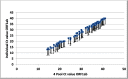
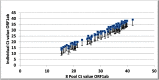
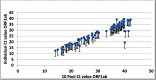
Sample pooling testing for SARS-COV-2 can be an effective tool in COVID-19 screening when resources are limited, yet it is important to assess the performance before implementation as pooling has its limitations. Our objective was to assess the efficacy of pooling samples for coronavirus 2019 (COVID-19) compared to an individual analysis by using commercial platforms for nucleic acid testing. A total of 2200 nasopharyngeal swabs for SARS-COV-2 were tested individually and in pools of 4, 8, and 10. The cycle threshold (Ct) values of the positive pooled samples were compared to their corresponding individual positive samples. In pool size 10 samples, an estimated increase of 3-Ct was obtained, which led to false negative results in low viral load positive samples. Pooling SARS COV-2 samples is an effective strategy of screening to increase laboratories' capacity and reduce costs without affecting diagnostic performance. A pool size of 8 is recommended.
Keywords: COVID-19; Limited resources; RT-PCR; SARS-COV-2; Sample pooling; Screening.
© The Author(s), under exclusive licence to Springer-Verlag London Ltd., part of Springer Nature 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures
Similar articles
-
Pooling Nasopharyngeal Swab Specimens to Improve Testing Capacity for SARS-CoV-2 by Real-Time RT-PCR.Biol Proced Online. 2021 Sep 30;23(1):19. doi: 10.1186/s12575-021-00156-6. Biol Proced Online. 2021. PMID: 34592917 Free PMC article.
-
Sample pooling for SARS-CoV-2 RT-PCR screening.Clin Microbiol Infect. 2020 Dec;26(12):1687.e1-1687.e5. doi: 10.1016/j.cmi.2020.09.008. Epub 2020 Sep 10. Clin Microbiol Infect. 2020. PMID: 32919074 Free PMC article.
-
Detection of SARS-CoV-2 RNA in Upper Respiratory Swap Samples by Pooling Method.Balkan Med J. 2022 Jan 25;39(1):48-54. doi: 10.5152/balkanmedj.2021.21135. Epub 2021 Dec 20. Balkan Med J. 2022. PMID: 34928231 Free PMC article.
-
Effectiveness of Sample Pooling Strategies for SARS-CoV-2 Mass Screening by RT-PCR: A Scoping Review.J Lab Physicians. 2020 Dec;12(3):212-218. doi: 10.1055/s-0040-1721159. Epub 2020 Nov 23. J Lab Physicians. 2020. PMID: 33268939 Free PMC article.
-
Test Groups, Not Individuals: A Review of the Pooling Approaches for SARS-CoV-2 Diagnosis.Diagnostics (Basel). 2021 Jan 4;11(1):68. doi: 10.3390/diagnostics11010068. Diagnostics (Basel). 2021. PMID: 33406644 Free PMC article. Review.
References
-
- Barak N, Ben-Ami R, Sido T, Perri A, Shtoyer A, Rivkin M, Licht T, Peretz A, Magenheim J, Fogel I, Livneh A, Daitch Y, Oiknine-Djian E, Benedek G, Dor Y, Wolf DG, Yassour M (2021) Hebrew University-Hadassah COVID-19 Diagnosis Team. Lessons from applied large-scale pooling of 133,816 SARS-CoV-2 RT-PCR tests. Sci Transl Med 13(589):eabf2823. 10.1126/scitranslmed.abf2823. Epub 2021 Feb 22. PMID: 33619081; PMCID: PMC8099176 - PMC - PubMed
-
- Barathidasan R, Sharmila FM, Raj RV, Dhanalakshmi G, Anitha G, Dhodapkar R (2022) Pooled sample testing for COVID-19 diagnosis: evaluation of bi-directional matrix pooling strategies. J Virol Methods 304:114524. 10.1016/j.jviromet.2022.114524. Epub 2022 Mar 15. PMID: 35301022; PMCID: PMC8920575 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
