Dual role of Apolipoprotein D as long-term instructive factor and acute signal conditioning microglial secretory and phagocytic responses
- PMID: 36779011
- PMCID: PMC9908747
- DOI: 10.3389/fncel.2023.1112930
Dual role of Apolipoprotein D as long-term instructive factor and acute signal conditioning microglial secretory and phagocytic responses
Abstract
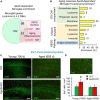
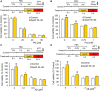
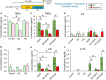
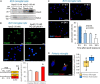
Microglial cells are recognized as very dynamic brain cells, screening the environment and sensitive to signals from all other cell types in health and disease. Apolipoprotein D (ApoD), a lipid-binding protein of the Lipocalin family, is required for nervous system optimal function and proper development and maintenance of key neural structures. ApoD has a cell and state-dependent expression in the healthy nervous system, and increases its expression upon aging, damage or neurodegeneration. An extensive overlap exists between processes where ApoD is involved and those where microglia have an active role. However, no study has analyzed the role of ApoD in microglial responses. In this work, we test the hypothesis that ApoD, as an extracellular signal, participates in the intercellular crosstalk sensed by microglia and impacts their responses upon physiological aging or damaging conditions. We find that a significant proportion of ApoD-dependent aging transcriptome are microglia-specific genes, and show that lack of ApoD in vivo dysregulates microglial density in mouse hippocampus in an age-dependent manner. Murine BV2 and primary microglia do not express ApoD, but it can be internalized and targeted to lysosomes, where unlike other cell types it is transiently present. Cytokine secretion profiles and myelin phagocytosis reveal that ApoD has both long-term pre-conditioning effects on microglia as well as acute effects on these microglial immune functions, without significant modification of cell survival. ApoD-triggered cytokine signatures are stimuli (paraquat vs. Aβ oligomers) and sex-dependent. Acute exposure to ApoD induces microglia to switch from their resting state to a secretory and less phagocytic phenotype, while long-term absence of ApoD leads to attenuated cytokine induction and increased myelin uptake, supporting a role for ApoD as priming or immune training factor. This knowledge should help to advance our understanding of the complex responses of microglia during aging and neurodegeneration, where signals received along our lifespan are combined with damage-triggered acute signals, conditioning both beneficial roles and limitations of microglial functions.
Keywords: acute response; amyloid-beta endocytosis; astrocyte-microglia crosstalk; cytokine secretion; immune memory; membrane-binding protein; microglia; myelin phagocytosis.
Copyright © 2023 Corraliza-Gomez, Bendito, Sandonis-Camarero, Mondejar-Duran, Villa, Poncela, Valero, Sanchez and Ganfornina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bajo-Grañeras R., Ganfornina M. D., Martín-Tejedor E., Sanchez D. (2011a). Apolipoprotein D mediates autocrine protection of astrocytes and controls their reactivity level, contributing to the functional maintenance of paraquat-challenged dopaminergic systems. Glia 59 1551–1566. 10.1002/glia.21200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

