Fulminant myocarditis with complete atrioventricular block after mRNA COVID-19 vaccination: A case report
- PMID: 36779079
- PMCID: PMC9906563
- DOI: 10.1016/j.jccase.2023.01.004
Fulminant myocarditis with complete atrioventricular block after mRNA COVID-19 vaccination: A case report
Abstract
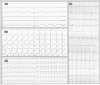
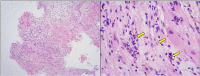
A 71-year-old man was transferred urgently to our hospital after collapsing near his home post the first shot of the BNT162b2 coronavirus disease 2019 vaccine (Pfizer-BioNTech, Comirnaty®). Immediately after arrival at our hospital, cardiac arrest due to complete atrioventricular block with no ventricular escaped beats was observed on electrocardiogram. Echocardiography showed preserved left ventricular ejection fraction, however, diffuse severe hypokinesia was revealed after 3 weeks, and he died 3 months after admission because of worsening heart failure. An autopsy examination revealed eosinophilic myocarditis or hypersensitivity myocarditis with extensive fibrosis and widespread myocardial dropout throughout the heart.
Learning objective: 1. Severe myocarditis occurs extremely rarely after mRNA coronavirus disease 2019 (COVID-19) vaccination. 2. Myocarditis after mRNA COVID-19 vaccination might cause complete atrioventricular block, followed by a course of decreased left ventricular ejection fraction. 3. Histologically, severe myocarditis after mRNA COVID-19 vaccination seems to present as fulminant necrotizing eosinophilic myocarditis or hypersensitivity myocarditis.
Keywords: BNT162b2; Complete atrioventricular block; Coronavirus disease 2019; Eosinophilic myocarditis; Hypersensitivity myocarditis; mRNA.
© 2023 Japanese College of Cardiology. Published by Elsevier Ltd.
Conflict of interest statement
None.
Figures

References
-
- Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., Edwards K., Soslow J.H., Dendy J.M., Schlaudecker E., Lang S.M., Barnett E.D., Ruberg F.L., Smith M.J., Campbell M.J., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. - PMC - PubMed
-
- Ameratunga R., Woon S.T., Sheppard M.N., Garland J., Ondruschka B., Wong C.X., Stewart R.A.H., Tatley M., Stables S.R., Tse R.D. First identified case of fatal fulminant eosinophilic myocarditis following the initial dose of the Pfizer-BioNTech mRNA COVID-19 vaccine (BNT162b2, Comirnaty): an extremely rare idiosyncratic hypersensitivity reaction. J Clin Immunol. 2022;42:441–447. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

