Modulation of Burn Hypermetabolism in Preclinical Models
- PMID: 36779088
- PMCID: PMC9904913
- DOI: 10.7759/cureus.33518
Modulation of Burn Hypermetabolism in Preclinical Models
Abstract
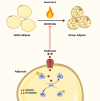
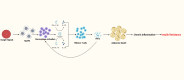
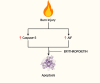
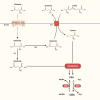
Severe burns elicit a state of physiological stress and increased metabolism to help the body compensate for the changes associated with the traumatic injury. However, this hypermetabolic state is associated with increased insulin resistance, cardiovascular dysfunction, skeletal muscle catabolism, impaired wound healing, and delayed recovery. Several interventions were attempted to modulate burn hypermetabolism, including nutritional support, early excision and grafting, and growth hormone application. However, burn hypermetabolism still imposes significant morbidity and mortality in burn patients. Due to the limitations of in vitro models, animal models are indispensable in burn research. Animal models provide researchers with invaluable tools to test the safety and efficacy of novel treatments or advance our knowledge of previously utilized agents. Several animal studies evaluated novel therapies to modulate burn hypermetabolism in the last few years, including recombinant human growth hormone, erythropoietin, acipimox, apelin, anti-interleukin-6 monoclonal antibody, and ghrelin therapies. Results from these studies are promising and may be effectively translated into human studies. In addition, other studies revisited drugs previously used in clinical practice, such as insulin and metformin, to further investigate their underlying mechanisms as modulators of burn hypermetabolism. This review aims to update burn experts with the novel therapies under investigation in burn hypermetabolism with a focus on applicability and translation. Furthermore, we aim to guide researchers in selecting the correct animal model for their experiments by providing a summary of the methodology and the rationale of the latest studies.
Keywords: animal models; burn; hypermetabolism; new therapeutics; review.
Copyright © 2023, Eldaly et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Nutritional and pharmacological modulation of the metabolic response of severely burned patients: review of the literature (part II)*. Atiyeh BS, Gunn SW, Dibo SA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3188173/ Ann Burns Fire Disasters. 2008;21:119–123. - PMC - PubMed
-
- The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. Yu YM, Tompkins RG, Ryan CM, Young VR. JPEN J Parenter Enteral Nutr. 1999;23:160–168. - PubMed
-
- Guillory AN, Porter C, Suman OE, Zapata-Sirvent RL, Finnerty CC, Herndon DN. Total Burn Care. 5th edition. Elsevier; 2018. Modulation of the hypermetabolic response after burn injury; pp. 301–306.
-
- Thermal injury. Ramzy PI, Barret JP, Herndon DN. Crit Care Clin. 1999;15:333–352. - PubMed
-
- Efficacy of a high-carbohydrate diet in catabolic illness. Hart DW, Wolf SE, Zhang XJ, et al. Crit Care Med. 2001;29:1318–1324. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
