Emergence of high-level colistin resistance mediated by multiple determinants, including mcr-1.1, mcr-8.2 and crrB mutations, combined with tigecycline resistance in an ST656 Klebsiella pneumoniae
- PMID: 36779188
- PMCID: PMC9909390
- DOI: 10.3389/fcimb.2023.1122532
Emergence of high-level colistin resistance mediated by multiple determinants, including mcr-1.1, mcr-8.2 and crrB mutations, combined with tigecycline resistance in an ST656 Klebsiella pneumoniae
Abstract
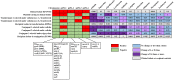
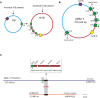
Colistin and tigecycline are usually regarded as the last resort for multidrug-resistant Klebsiella pneumoniae infection treatment. Emergence of colistin and tigecycline resistance poses a global healthcare challenge and is associated with high mortality due to limited therapeutic options. Here, we report the ST656 extensively drug-resistant K. pneumoniae strain KP15-652, which was isolated from a patient's urine in China. Antimicrobial susceptibility testing showed it to be resistant to tigecycline, amikacin, levofloxacin, ciprofloxacin, and high-level colistin resistance (> 2048 mg/L). Whole-genome sequencing revealed that it harbors one chromosome and seven plasmids, including four plasmids carrying multiple acquired resistance genes. Transformation/conjugation tests and plasmid curing assays confirmed that mcr-1.1, mcr-8.2 and crrB mutations are responsible for the high-level colistin resistance and that a series of efflux pump genes, such as tmexCD1-toprJ1, tet(A) and tet(M), contribute to tigecycline resistance. mcr-1.1 and tet(M) are located on an IncX1 plasmid, which has conjugation transfer potential. mcr-8.2 and tet(A) are located on a multireplicon IncR/IncN plasmid but unable to be transferred via conjugation. Moreover, another conjugable and fusion plasmid carries the tmexCD1-toprJ1 gene cluster, which may have arisen due to IS26-mediated replicative transposition based on 8-bp target-site duplications. Importantly, a complex class 1 integron carrying various resistance genes was detected on this fusion plasmid. In conclusion, it is possible that the high-level of colistin resistance is caused by the accumulated effect of several factors on the chromosome and mcr-carrying plasmids, combined with many other resistances, including tigecycline. Effective surveillance should be performed to prevent further dissemination.
Keywords: co-integration; colistin; mcr; tigecycline; tmexCD1-toprJ1.
Copyright © 2023 Wang, Zhou, Liu, Wang, Zhang, Zhu, Zhao, Wu, Yu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Co-transfer of IncFII/IncFIB and IncFII plasmids mediated by IS26 facilitates the transmission of mcr-8.1 and tmexCD1-toprJ1.Ann Clin Microbiol Antimicrob. 2024 Feb 13;23(1):14. doi: 10.1186/s12941-024-00676-5. Ann Clin Microbiol Antimicrob. 2024. PMID: 38350903 Free PMC article.
-
Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae.Emerg Microbes Infect. 2020 Dec;9(1):1102-1113. doi: 10.1080/22221751.2020.1768805. Emerg Microbes Infect. 2020. PMID: 32401163 Free PMC article.
-
Co-existence of two plasmids harboring transferable resistance-nodulation-division pump gene cluster, tmexCD1-toprJ1, and colistin resistance gene mcr-8 in Klebsiella pneumoniae.Ann Clin Microbiol Antimicrob. 2024 Jul 26;23(1):67. doi: 10.1186/s12941-024-00727-x. Ann Clin Microbiol Antimicrob. 2024. PMID: 39061085 Free PMC article.
-
New tools to mitigate drug resistance in Enterobacteriaceae - Escherichia coli and Klebsiella pneumoniae.Crit Rev Microbiol. 2023 Aug;49(4):435-454. doi: 10.1080/1040841X.2022.2080525. Epub 2022 Jun 1. Crit Rev Microbiol. 2023. PMID: 35649163 Review.
-
Gram-negative bacilli carrying mcr gene in Brazil: a pathogen on the rise.Braz J Microbiol. 2023 Jun;54(2):1009-1020. doi: 10.1007/s42770-023-00948-w. Epub 2023 Mar 21. Braz J Microbiol. 2023. PMID: 36943639 Free PMC article. Review.
Cited by
-
Convergence of plasmid-mediated Colistin and Tigecycline resistance in Klebsiella pneumoniae.Front Microbiol. 2024 Jan 3;14:1221428. doi: 10.3389/fmicb.2023.1221428. eCollection 2023. Front Microbiol. 2024. PMID: 38282729 Free PMC article.
-
One Health at Risk: Plasmid-Mediated Spread of mcr-1 Across Clinical, Agricultural, and Environmental Ecosystems.Antibiotics (Basel). 2025 May 15;14(5):506. doi: 10.3390/antibiotics14050506. Antibiotics (Basel). 2025. PMID: 40426572 Free PMC article. Review.
-
Co-transfer of IncFII/IncFIB and IncFII plasmids mediated by IS26 facilitates the transmission of mcr-8.1 and tmexCD1-toprJ1.Ann Clin Microbiol Antimicrob. 2024 Feb 13;23(1):14. doi: 10.1186/s12941-024-00676-5. Ann Clin Microbiol Antimicrob. 2024. PMID: 38350903 Free PMC article.
-
Genomic analysis of carbapenem- and colistin-resistant Klebsiella pneumoniae complex harbouring mcr-8 and mcr-9 from individuals in Thailand.Sci Rep. 2024 Jul 22;14(1):16836. doi: 10.1038/s41598-024-67838-5. Sci Rep. 2024. PMID: 39039157 Free PMC article.
References
-
- Dong N., Zeng Y., Wang Y., Liu C., Lu J., Cai C., et al. . (2022). Distribution and spread of the mobilised RND efflux pump gene cluster tmexCD-toprJ in clinical gram-negative bacteria: a molecular epidemiological study. Lancet Microbe 3 (11), e846–e856. doi: 10.1016/S2666-5247(22)00221-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

