A zone-of-inhibition assay to screen for humoral antimicrobial activity in mosquito hemolymph
- PMID: 36779191
- PMCID: PMC9908765
- DOI: 10.3389/fcimb.2023.891577
A zone-of-inhibition assay to screen for humoral antimicrobial activity in mosquito hemolymph
Abstract
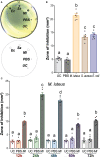
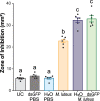
In insects, antibacterial immunity largely depends on the activation of downstream signaling and effector responses, leading to the synthesis and secretion of soluble effector molecules, such as antimicrobial peptides (AMPs). AMPs are acute infection response peptides secreted into the hemolymph upon bacterial stimulation. The transcription of innate immunity genes encoding for AMPs is highly dependent on several signaling cascade pathways, such as the Toll pathway. In the African malaria mosquito, Anopheles gambiae, AMPs hold a special interest as their upregulation have been shown to limit the growth of malaria parasites, bacteria, and fungi. Most of the current knowledge on the regulation of insect AMPs in microbial infection have been obtained from Drosophila. However, largely due to the lack of convenient assays, the regulation of antimicrobial activity in mosquito hemolymph is still not completely understood. In this study, we report a zone of inhibition assay to identify the contribution of AMPs and components of the Toll pathway to the antimicrobial activity of A. gambiae hemolymph. As a proof of principle, we demonstrate that Micrococcus luteus challenge induces antimicrobial activity in the adult female mosquito hemolymph, which is largely dependent on defensin 1. Moreover, by using RNAi to silence Cactus, REL1, and MyD88, we showed that Cactus kd induces antimicrobial activity in the mosquito hemolymph, whereas the antimicrobial activity in REL1 kd and MyD88 kd is reduced after challenge. Finally, while injection itself is not sufficient to induce antimicrobial activity, our results show that it primes the response to bacterial challenge. Our study provides information that increases our knowledge of the regulation of antimicrobial activity in response to microbial infections in mosquitoes. Furthermore, this assay represents an ex vivo medium throughput assay that can be used to determine the upstream regulatory elements of antimicrobial activity in A. gambiae hemolymph.
Keywords: ZOI (zone of inhibition); antimicrobial peptide (AMP); defensin 1; innate immunity; mosquito; toll pathway.
Copyright © 2023 Morejon and Michel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


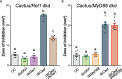
Similar articles
-
The Route of Infection Influences the Contribution of Key Immunity Genes to Antibacterial Defense in Anopheles gambiae.J Innate Immun. 2021;13(2):107-126. doi: 10.1159/000511401. Epub 2020 Nov 18. J Innate Immun. 2021. PMID: 33207342 Free PMC article.
-
The defensin peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes.Insect Biochem Mol Biol. 2001 Mar 1;31(3):241-8. doi: 10.1016/s0965-1748(00)00143-0. Insect Biochem Mol Biol. 2001. PMID: 11167093
-
The serine protease homolog CLIPA14 modulates the intensity of the immune response in the mosquito Anopheles gambiae.J Biol Chem. 2017 Nov 3;292(44):18217-18226. doi: 10.1074/jbc.M117.797787. Epub 2017 Sep 19. J Biol Chem. 2017. PMID: 28928218 Free PMC article.
-
NF-κB-Like Signaling Pathway REL2 in Immune Defenses of the Malaria Vector Anopheles gambiae.Front Cell Infect Microbiol. 2017 Jun 21;7:258. doi: 10.3389/fcimb.2017.00258. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680852 Free PMC article. Review.
-
Serine proteases as mediators of mosquito immune responses.Insect Biochem Mol Biol. 2001 Mar 1;31(3):257-62. doi: 10.1016/s0965-1748(00)00145-4. Insect Biochem Mol Biol. 2001. PMID: 11167095 Review.
Cited by
-
CLIPB4 is a central node in the protease network that regulates humoral immunity in Anopheles gambiae mosquitoes.bioRxiv [Preprint]. 2023 Jul 16:2023.07.07.545904. doi: 10.1101/2023.07.07.545904. bioRxiv. 2023. Update in: J Innate Immun. 2023;15(1):680-696. doi: 10.1159/000533898. PMID: 37461554 Free PMC article. Updated. Preprint.
-
Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus.Int J Mol Sci. 2023 Dec 17;24(24):17565. doi: 10.3390/ijms242417565. Int J Mol Sci. 2023. PMID: 38139394 Free PMC article.
-
Synthesis and photocatalytic degradation of azithromycin by iron/zinc oxide nanoparticle-reinforced carbon nanofibers.Sci Rep. 2025 Aug 25;15(1):31203. doi: 10.1038/s41598-025-16849-x. Sci Rep. 2025. PMID: 40855187 Free PMC article.
-
The expanded immunoregulatory protease network in mosquitoes is governed by gene co-expression.bioRxiv [Preprint]. 2024 Aug 4:2024.06.18.599423. doi: 10.1101/2024.06.18.599423. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 May 6;122(18):e2425863122. doi: 10.1073/pnas.2425863122. PMID: 38948760 Free PMC article. Updated. Preprint.
-
The expanded immunoregulatory protease network in mosquitoes is governed by gene coexpression.Proc Natl Acad Sci U S A. 2025 May 6;122(18):e2425863122. doi: 10.1073/pnas.2425863122. Epub 2025 Apr 30. Proc Natl Acad Sci U S A. 2025. PMID: 40305045
References
-
- Andersons D., Gunne H., Hellers M., Johansson H., Steiner H. (1990). Immune responses in Trichoplusia ni challenged with bacteria or baculoviruses. Insect Biochem. 20, 537–543. doi: 10.1016/0020-1790(90)90037-U - DOI
-
- Apidianakis Y., Mindrinos M. N., Xiao W., Lau G. W., Baldini R. L., Davis R. W., et al. . (2005). Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. PNAS 102, 2573–2578. doi: 10.1073/pnas.0409588102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

