Changing Cerebral Blood Flow, Glucose Metabolism, and Dopamine Binding Through Transcranial Magnetic Stimulation: A Systematic Review of Transcranial Magnetic Stimulation-Positron Emission Tomography Literature
- PMID: 36779330
- PMCID: PMC9580100
- DOI: 10.1124/pharmrev.122.000579
Changing Cerebral Blood Flow, Glucose Metabolism, and Dopamine Binding Through Transcranial Magnetic Stimulation: A Systematic Review of Transcranial Magnetic Stimulation-Positron Emission Tomography Literature
Abstract
Transcranial magnetic stimulation (TMS) is a noninvasive neuromodulation tool currently used as a treatment in multiple psychiatric and neurologic disorders. Despite its widespread use, we have an incomplete understanding of the way in which acute and chronic sessions of TMS affect various neural and vascular systems. This systematic review summarizes the state of our knowledge regarding the effects TMS may be having on cerebral blood flow, glucose metabolism, and neurotransmitter release. Forty-five studies were identified. Several key themes emerged: 1) TMS transiently increases cerebral blood flow in the area under the coil; 2) TMS to the prefrontal cortex increases glucose metabolism in the anterior cingulate cortex of patients with depression; and 3) TMS to the motor cortex and prefrontal cortex decreases dopamine receptor availability in the ipsilateral putamen and caudate respectively. There is, however, a paucity of literature regarding the effects TMS may have on other neurotransmitter and neuropeptide systems of interest, all of which may shed vital light on existing biologic mechanisms and future therapeutic development. SIGNIFICANCE STATEMENT: Transcranial magnetic stimulation (TMS) is a noninvasive neuromodulation tool currently used as a treatment in multiple psychiatric and neurologic disorders. This systematic review summarizes the state of our knowledge regarding the effects TMS on cerebral blood flow, glucose metabolism, and neurotransmitter release.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
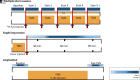

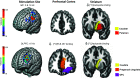
References
-
- Adachi YU, Yamada S, Satomoto M, Watanabe K, Higuchi H, Kazama T, Doi M, Sato S (2006) Pentobarbital inhibits L-DOPA-induced dopamine increases in the rat striatum: An in vivo microdialysis study. Brain Res Bull 69:593–596. - PubMed
-
- Baeken C, De Raedt R, Van Hove C, Clerinx P, De Mey J, Bossuyt A (2009) HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS Spectr 14:439–448. - PubMed
-
- Baeken C, Marinazzo D, Everaert H, Wu GR, Van Hove C, Audenaert K, Goethals I, De Vos F, Peremans K, De Raedt R (2015) The Impact of Accelerated HF-rTMS on the Subgenual Anterior Cingulate Cortex in Refractory Unipolar Major Depression: Insights From 18FDG PET Brain Imaging. Brain Stimul 8:808–815. - PubMed
-
- Bailey DL, Townsend DW, Valk PE, Maisey MN (2005) Positron Emission Tomography, 1st ed, Springer, London.
-
- Barrett J, Della-Maggiore V, Chouinard PA, Paus T (2004) Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and brain imaging studies. Neuropsychopharmacology 29:1172–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

