Narirutin activates TFEB (transcription factor EB) to protect against Acetaminophen-induced liver injury by targeting PPP3/calcineurin
- PMID: 36779633
- PMCID: PMC10351474
- DOI: 10.1080/15548627.2023.2179781
Narirutin activates TFEB (transcription factor EB) to protect against Acetaminophen-induced liver injury by targeting PPP3/calcineurin
Abstract
Acetaminophen (APAP) overdose is the predominant cause of drug-induced liver injury worldwide. The macroautophagy/autophagy-lysosomal pathway (ALP) is involved in the APAP hepatotoxicity. TFEB (transcription factor EB) promotes the expression of genes related to autophagy and lysosomal biogenesis, thus, pharmacological activation of TFEB-mediated ALP may be an effective therapeutic approach for treating APAP-induced liver injury. We aimed to reveal the effects of narirutin (NR), the main bioactive constituents isolated from citrus peels, on APAP hepatotoxicity and to explore its underlying mechanism. Administration of NR enhanced activities of antioxidant enzymes, improved mitochondrial dysfunction and alleviated liver injury in APAP-treated mice, whereas NR did not affect APAP metabolism and MAPK/JNK activation. NR enhanced TFEB transcriptional activity and activated ALP in an MTOR complex 1 (MTORC1)-independent but PPP3/calcineurin-dependent manner. Moreover, knockout of Tfeb or knockdown of PPP3CB/CNA2 (protein phosphatase 3, catalytic subunit, beta isoform) in the liver abolished the beneficial effects of NR on APAP overdose. Mechanistically, NR bound to PPP3CB via PRO31, LYS61 and PRO347 residues and enhanced PPP3/calcineurin activity, thereby eliciting dephosphorylation of TFEB and promoting ALP, which alleviated APAP-induced oxidative stress and liver injury. Together, NR protects against APAP-induced liver injury by activating a PPP3/calcineurin-TFEB-ALP axis, indicating NR may be a potential agent for treating APAP overdose.Abbreviations: ALP: autophagy-lysosomal pathway; APAP: acetaminophen; APAP-AD: APAP-protein adducts; APAP-Cys: acetaminophen-cysteine adducts; CAT: catalase; CETSA: cellular thermal shift assay; CQ: chloroquine; CYP2E1: cytochrome P450, family 2, subfamily e, polypeptide 1; CYCS/Cyt c: cytochrome c, somatic; DARTS: drug affinity responsive target stability assay; ENGASE/NAG: endo-beta-N-acetylglucosaminidase; GOT1/AST: glutamic-oxaloacetic transaminase 1, soluble; GPT/ALT: glutamic pyruvic transaminase, soluble; GSH: glutathione; GPX/GSH-Px: glutathione peroxidase; KD: dissociation constant; Leu: leupeptin; MCOLN1: mucolipin 1; MTORC1: MTOR complex 1; NAC: N-acetylcysteine; NAPQI: N-acetyl-p-benzoquinoneimine; NFAT: nuclear factor of activated T cells; NR: narirutin; OA: okadaic acid; RRAG: Ras related GTP binding; ROS: reactive oxygen species; PPP3CB/CNA2: protein phosphatase 3, catalytic subunit, beta isoform; PPP3R1/CNB1: protein phosphatase 3, regulatory subunit B, alpha isoform (calcineurin B, type I); SOD: superoxide dismutase; SPR: surface plasmon resonance analysis; TFEB: transcription factor EB.
Keywords: PPP3CB/CNA2; TFEB; autophagy-lysosomal pathway; hepatotoxicity; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures


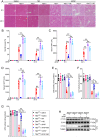
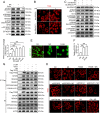



References
-
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11(3):525–548. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
