Renoprotective effects of empagliflozin are linked to activation of the tubuloglomerular feedback mechanism and blunting of the complement system
- PMID: 36779666
- PMCID: PMC10085567
- DOI: 10.1152/ajpcell.00528.2022
Renoprotective effects of empagliflozin are linked to activation of the tubuloglomerular feedback mechanism and blunting of the complement system
Abstract
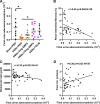
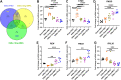
The mechanisms of nephroprotection in nondiabetic chronic kidney disease (CKD) models by sodium-glucose cotransporter 2 (SGLT2) inhibitors are not well defined. Five groups were established: sham-operated rats, placebo-treated rats with 5/6 nephrectomy (5/6Nx), 5/6Nx + telmisartan (5 mg/kg/day), 5/6Nx + empagliflozin (3 mg/kg/day), and 5/6Nx + empagliflozin (15 mg/kg/day). Treatment duration was 95 days. Empagliflozin showed a dose-dependent beneficial effect on the change from baseline of creatinine clearance (Ccr). The urinary albumin-to-creatinine ratio likewise improved in a dose-dependent manner. Both dosages of empagliflozin improved morphological kidney damage parameters such as renal interstitial fibrosis and glomerulosclerosis. 5/6 nephrectomy led to a substantial reduction of urinary adenosine excretion, a surrogate parameter of the tubuloglomerular feedback (TGF) mechanism. Empagliflozin caused a dose-dependent increase in urinary adenosine excretion. The urinary adenosine excretion was negatively correlated with renal interstitial fibrosis and positively correlated with Ccr. Immunofluorescence analysis revealed that empagliflozin had no effect on CD8+ and CD4+ T cells as well as on CD68+ cells (macrophages). To further explore potential mechanisms, a nonhypothesis-driven approach was used. RNA sequencing followed by quantitative real-time polymerase chain reaction revealed that complement component 1Q subcomponent A chain (C1QA) as well as complement component 1Q subcomponent C chain (C1QC) gene expression were upregulated in the placebo-treated 5/6Nx rats and this upregulation was blunted by treatment with empagliflozin. In conclusion, empagliflozin-mediated nephroprotection in nondiabetic CKD is due to a dose-dependent activation of the TGF as well as empagliflozin-mediated effects on the complement system.
Keywords: complement system; nondiabetic chronic kidney disease; sodium-glucose cotransporter 2 inhibitor; tubuloglomerular feedback.
Conflict of interest statement
B.K.K. reports lecture fees and/or advisory board memberships and/or study participation from Astellas, Bayer, Boehringer Ingelheim, Chiesi, Riepharm, Pfizer, Sanofi, Servier and Vifor Pharma, all not related to the submitted work. D.D. and T.K. are research employees of Boehringer Ingelheim. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




Similar articles
-
Antifibrotic effects of low dose SGLT2 Inhibition with empagliflozin in comparison to Ang II receptor blockade with telmisartan in 5/6 nephrectomised rats on high salt diet.Biomed Pharmacother. 2022 Feb;146:112606. doi: 10.1016/j.biopha.2021.112606. Epub 2021 Dec 28. Biomed Pharmacother. 2022. PMID: 34968924
-
Empagliflozin reduces kidney fibrosis and improves kidney function by alternative macrophage activation in rats with 5/6-nephrectomy.Biomed Pharmacother. 2022 Dec;156:113947. doi: 10.1016/j.biopha.2022.113947. Epub 2022 Oct 31. Biomed Pharmacother. 2022. PMID: 36411661
-
SGLT2 inhibition promotes glomerular repopulation by cells of renin lineage in experimental kidney disease.Acta Physiol (Oxf). 2024 Mar;240(3):e14108. doi: 10.1111/apha.14108. Epub 2024 Feb 5. Acta Physiol (Oxf). 2024. PMID: 38314444 Free PMC article.
-
Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Non-Diabetic Chronic Kidney Disease.Adv Ther. 2021 May;38(5):2201-2212. doi: 10.1007/s12325-021-01735-5. Epub 2021 Apr 16. Adv Ther. 2021. PMID: 33860925 Review.
-
Sodium-glucose cotransporter inhibitors and kidney fibrosis: review of the current evidence and related mechanisms.Pharmacol Rep. 2023 Feb;75(1):44-68. doi: 10.1007/s43440-022-00442-4. Epub 2022 Dec 19. Pharmacol Rep. 2023. PMID: 36534320 Review.
Cited by
-
Complement Immune System in Pulmonary Hypertension-Cooperating Roles of Circadian Rhythmicity in Complement-Mediated Vascular Pathology.Int J Mol Sci. 2024 Nov 28;25(23):12823. doi: 10.3390/ijms252312823. Int J Mol Sci. 2024. PMID: 39684535 Free PMC article. Review.
-
Complement anaphylatoxins: Potential therapeutic target for diabetic kidney disease.Diabet Med. 2025 Feb;42(2):e15427. doi: 10.1111/dme.15427. Epub 2024 Aug 27. Diabet Med. 2025. PMID: 39189098 Free PMC article. Review.
-
GSDMD Mediates Ang II-Induced Hypertensive Nephropathy by Regulating the GATA2/AQP4 Signaling Pathway.J Inflamm Res. 2024 Nov 5;17:8241-8259. doi: 10.2147/JIR.S488553. eCollection 2024. J Inflamm Res. 2024. PMID: 39525316 Free PMC article.
-
Nascent shifts in renal cellular metabolism, structure, and function due to chronic empagliflozin in prediabetic mice.Am J Physiol Cell Physiol. 2024 Apr 1;326(4):C1272-C1290. doi: 10.1152/ajpcell.00446.2023. Epub 2024 Mar 4. Am J Physiol Cell Physiol. 2024. PMID: 38602847 Free PMC article.
-
Kidney Fibrosis and Oxidative Stress: From Molecular Pathways to New Pharmacological Opportunities.Biomolecules. 2024 Jan 22;14(1):137. doi: 10.3390/biom14010137. Biomolecules. 2024. PMID: 38275766 Free PMC article. Review.
References
-
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013. doi:10.7326/0003-4819-158-11-201306040-00007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

