Staphylococcus aureus Prophage-Encoded Protein Causes Abortive Infection and Provides Population Immunity against Kayviruses
- PMID: 36779718
- PMCID: PMC10127798
- DOI: 10.1128/mbio.02490-22
Staphylococcus aureus Prophage-Encoded Protein Causes Abortive Infection and Provides Population Immunity against Kayviruses
Abstract
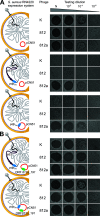
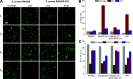
Both temperate and obligately lytic phages have crucial roles in the biology of staphylococci. While superinfection exclusion among closely related temperate phages is a well-characterized phenomenon, the interactions between temperate and lytic phages in staphylococci are not understood. Here, we present a resistance mechanism toward lytic phages of the genus Kayvirus, mediated by the membrane-anchored protein designated PdpSau encoded by Staphylococcus aureus prophages, mostly of the Sa2 integrase type. The prophage accessory gene pdpSau is strongly linked to the lytic genes for holin and ami2-type amidase and typically replaces genes for the toxin Panton-Valentine leukocidin (PVL). The predicted PdpSau protein structure shows the presence of a membrane-binding α-helix in its N-terminal part and a cytoplasmic positively charged C terminus. We demonstrated that the mechanism of action of PdpSau does not prevent the infecting kayvirus from adsorbing onto the host cell and delivering its genome into the cell, but phage DNA replication is halted. Changes in the cell membrane polarity and permeability were observed from 10 min after the infection, which led to prophage-activated cell death. Furthermore, we describe a mechanism of overcoming this resistance in a host-range Kayvirus mutant, which was selected on an S. aureus strain harboring prophage 53 encoding PdpSau, and in which a chimeric gene product emerged via adaptive laboratory evolution. This first case of staphylococcal interfamily phage-phage competition is analogous to some other abortive infection defense systems and to systems based on membrane-destructive proteins. IMPORTANCE Prophages play an important role in virulence, pathogenesis, and host preference, as well as in horizontal gene transfer in staphylococci. In contrast, broad-host-range lytic staphylococcal kayviruses lyse most S. aureus strains, and scientists worldwide have come to believe that the use of such phages will be successful for treating and preventing bacterial diseases. The effectiveness of phage therapy is complicated by bacterial resistance, whose mechanisms related to therapeutic staphylococcal phages are not understood in detail. In this work, we describe a resistance mechanism targeting kayviruses that is encoded by a prophage. We conclude that the defense mechanism belongs to a broader group of abortive infections, which is characterized by suicidal behavior of infected cells that are unable to produce phage progeny, thus ensuring the survival of the host population. Since the majority of staphylococcal strains are lysogenic, our findings are relevant for the advancement of phage therapy.
Keywords: Kayvirus; Staphylococcus aureus; abortive infection; bacteriophage evolution; bacteriophage therapy; bacteriophages; cell death; lysogeny; phage resistance; phage therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Global Transcriptomic Analysis of Bacteriophage-Host Interactions between a Kayvirus Therapeutic Phage and Staphylococcus aureus.Microbiol Spectr. 2022 Jun 29;10(3):e0012322. doi: 10.1128/spectrum.00123-22. Epub 2022 Apr 18. Microbiol Spectr. 2022. PMID: 35435752 Free PMC article.
-
Characterization of a novel Phietavirus genus bacteriophage and its potential for efficient transfer of modified shuttle plasmids to Staphylococcus aureus strains of different clonal complexes.Microbiol Spectr. 2025 Aug 5;13(8):e0333224. doi: 10.1128/spectrum.03332-24. Epub 2025 Jul 11. Microbiol Spectr. 2025. PMID: 40642983 Free PMC article.
-
Efficient plasmid transduction to Staphylococcus aureus strains insensitive to the lytic action of transducing phage.FEMS Microbiol Lett. 2016 Oct;363(19):fnw211. doi: 10.1093/femsle/fnw211. Epub 2016 Sep 8. FEMS Microbiol Lett. 2016. PMID: 27609232
-
The Role of hlb-Converting Bacteriophages in Staphylococcus aureus Host Adaption.Microb Physiol. 2021;31(2):109-122. doi: 10.1159/000516645. Epub 2021 Jun 14. Microb Physiol. 2021. PMID: 34126612 Review.
-
The phages of staphylococci: critical catalysts in health and disease.Trends Microbiol. 2021 Dec;29(12):1117-1129. doi: 10.1016/j.tim.2021.04.008. Epub 2021 May 21. Trends Microbiol. 2021. PMID: 34030968 Free PMC article. Review.
Cited by
-
Bacteriophages avoid autoimmunity from cognate immune systems as an intrinsic part of their life cycles.Nat Microbiol. 2024 May;9(5):1312-1324. doi: 10.1038/s41564-024-01661-6. Epub 2024 Apr 2. Nat Microbiol. 2024. PMID: 38565896 Free PMC article.
-
Characterisation of Staphylococcus aureus Strains and Their Prophages That Carry Horse-Specific Leukocidin Genes lukP/Q.Toxins (Basel). 2025 Jan 3;17(1):20. doi: 10.3390/toxins17010020. Toxins (Basel). 2025. PMID: 39852974 Free PMC article.
-
Potential of training of anti-Staphylococcus aureus therapeutic phages against Staphylococcus epidermidis multidrug-resistant isolates is restricted by inter- and intra-sequence type specificity.mSystems. 2024 Oct 22;9(10):e0085024. doi: 10.1128/msystems.00850-24. Epub 2024 Sep 9. mSystems. 2024. PMID: 39248470 Free PMC article.
-
Phage therapy: A novel approach against multidrug-resistant pathogens.3 Biotech. 2024 Oct;14(10):256. doi: 10.1007/s13205-024-04101-8. Epub 2024 Sep 30. 3 Biotech. 2024. PMID: 39355200 Free PMC article. Review.
-
Effect of temperate bacteriophage vB_SauS_S1 on the adaptability and pathogenicity of Staphylococcus aureus ST398.BMC Microbiol. 2025 Mar 31;25(1):184. doi: 10.1186/s12866-025-03900-0. BMC Microbiol. 2025. PMID: 40165043 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

