Baculovirus Actin Rearrangement-Inducing Factor 1 Can Remodel the Mammalian Actin Cytoskeleton
- PMID: 36779726
- PMCID: PMC10100760
- DOI: 10.1128/spectrum.05189-22
Baculovirus Actin Rearrangement-Inducing Factor 1 Can Remodel the Mammalian Actin Cytoskeleton
Abstract
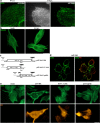
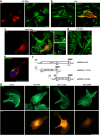
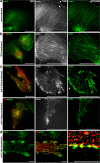
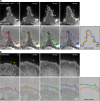
The actin rearrangement-inducing factor 1 (Arif-1) of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is an early viral protein that manipulates the actin cytoskeleton of host insect cells. Arif-1 is conserved among alphabaculoviruses and is responsible for the accumulation of F-actin at the plasma membrane during the early phase of infection. However, the molecular mechanism underlying Arif-1-induced cortical actin accumulation is still open. Recent studies have demonstrated the formation of invadosome-like structures induced by Arif-1, suggesting a function in systemic virus spread. Here, we addressed whether Arif-1 is able to manipulate the actin cytoskeleton of mammalian cells comparably to insect cells. Strikingly, transient overexpression of Arif-1 in B16-F1 mouse melanoma cells revealed pronounced F-actin remodeling. Actin assembly was increased, and intense membrane ruffling occurred at the expense of substrate-associated lamellipodia. Deletion mutagenesis studies of Arif-1 confirmed that the C-terminal cytoplasmic region was not sufficient to induce F-actin remodeling, supporting that the transmembrane region for Arif-1 function is also required in mammalian cells. The similarities between Arif-1-induced actin remodeling in insect and mammalian cells indicate that Arif-1 function relies on conserved cellular interaction partners and signal transduction pathways, thus providing an experimental tool to elucidate the underlying mechanism. IMPORTANCE Virus-induced changes of the host cell cytoskeleton play a pivotal role in the pathogenesis of viral infections. The baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is known for intervening with the regulation of the host actin cytoskeleton in a wide manner throughout the infection cycle. The actin rearrangement-inducing factor 1 (Arif-1) is a viral protein that causes actin rearrangement during the early phase of AcMNPV infection. Here, we performed overexpression studies of Arif-1 in mammalian cells to establish an experimental tool that allows elucidation of the mechanism underlying the Arif-1-induced remodeling of actin dynamics in a well-characterized and genetically accessible system.
Keywords: AcMNPV; Arif-1; B16-F1 cells; F-actin remodeling; TN368 cells; actin dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

