Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone
- PMID: 36779854
- PMCID: PMC10005765
- DOI: 10.7554/eLife.82112
Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone
Abstract
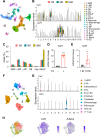
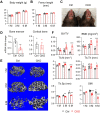
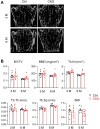
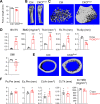
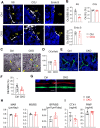
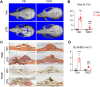
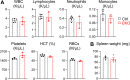
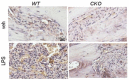
Colony-stimulating factor 1 (Csf1) is an essential growth factor for osteoclast progenitors and an important regulator for bone resorption. It remains elusive which mesenchymal cells synthesize Csf1 to stimulate osteoclastogenesis. We recently identified a novel mesenchymal cell population, marrow adipogenic lineage precursors (MALPs), in bone. Compared to other mesenchymal subpopulations, MALPs expressed Csf1 at a much higher level and this expression was further increased during aging. To investigate its role, we constructed MALP-deficient Csf1 CKO mice using AdipoqCre. These mice had increased femoral trabecular bone mass, but their cortical bone appeared normal. In comparison, depletion of Csf1 in the entire mesenchymal lineage using Prrx1Cre led to a more striking high bone mass phenotype, suggesting that additional mesenchymal subpopulations secrete Csf1. TRAP staining revealed diminished osteoclasts in the femoral secondary spongiosa region of Csf1 CKOAdipoq mice, but not at the chondral-osseous junction nor at the endosteal surface of cortical bone. Moreover, Csf1 CKOAdipoq mice were resistant to LPS-induced calvarial osteolysis. Bone marrow cellularity, hematopoietic progenitors, and macrophages were also reduced in these mice. Taken together, our studies demonstrate that MALPs synthesize Csf1 to control bone remodeling and hematopoiesis.
Keywords: Csf1; bone; cell biology; hematopoiesis; marrow adipogenic lineage precursors; medicine; mouse; osteoclasts.
© 2023, Zhong et al.
Conflict of interest statement
LZ, JL, JF, LY, WY, TG, MD, NH, CB, XH, SB, KT, CC, YC, SY, WT, ND, LQ No competing interests declared, JJ Reviewing editor, eLife
Figures






Update of
- doi: 10.1101/2022.07.27.501742
References
-
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-fms and receptor activator of nuclear factor kappaB (RANK) receptors. The Journal of Experimental Medicine. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. - DOI - PMC - PubMed
-
- Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, Dionne D, Papazian A, Lee D, Ashenberg O, Subramanian A, Vaishnav ED, Rozenblatt-Rosen O, Regev A, Scadden DT. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932. doi: 10.1016/j.cell.2019.04.040. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

