Validation of saline, PBS and a locally produced VTM at varying storage conditions to detect the SARS-CoV-2 virus by qRT-PCR
- PMID: 36780469
- PMCID: PMC9924993
- DOI: 10.1371/journal.pone.0280685
Validation of saline, PBS and a locally produced VTM at varying storage conditions to detect the SARS-CoV-2 virus by qRT-PCR
Abstract
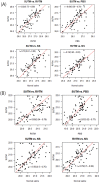
Coronavirus Disease-2019 tests require a Nasopharyngeal (NP) and/or Oropharyngeal (OP) specimen from the upper airway, from which virus RNA is extracted and detected through quantitative reverse transcription-Polymerase Chain Reaction (qRT-PCR). The viability of the virus is maintained after collection by storing the NP/OP swabs in Viral Transport Media (VTM). We evaluated the performance of four transport media: locally manufactured ("REVITAL") Viral Transport Media (RVTM), Standard Universal Transport Media (SUTM), PBS and 0.9% (w/v) NaCl (normal saline). We used laboratory cultured virus to evaluate: i) viral recovery and maintaining integrity at different time periods and temperatures; ii) stability in yielding detectable RNA consistently for all time points and conditions; and iii) their overall accuracy. Four vials of SARS-CoV-2 cultured virus (2 high and 2 low concentration samples) and 1 negative control sample were prepared for each media type (SUTM, RVTM, PBS and normal saline) and stored at the following temperatures, -80°C, 4°C, 25°C and 37°C for 7 days. Viral RNA extractions and qRT-PCR were performed at 1, 2, 3, 4 and 7 days after inoculation with the cultured virus to assess virus stability and viral recovery. Ct values fell over time at 25°C and 37°C, but normal saline, PBS, RVTM and SUTM all showed comparable performance in maintaining virus integrity and stability allowing for the detection of SARS-CoV-2 RNA. Overall, this study demonstrated that normal saline, PBS and the locally manufactured VTM can be used for COVID-19 sample collection and testing, thus expanding the range of SARS-CoV-2 viral collection media.
Copyright: © 2023 Ngetsa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Barrera-Avalos C, Luraschi R, Vallejos-Vidal E, Figueroa M, Arenillas E, Barría D, et al.. Analysis by real-time PCR of five transport and conservation mediums of nasopharyngeal swab samples to COVID-19 diagnosis in Santiago of Chile. J Med Virol. 2022. Mar 1;94(3):1167–74. doi: 10.1002/jmv.27446 - DOI - PMC - PubMed
-
- CDC. Centers for Disease Control and Prevention. Preparation of viral transport medium [Internet]. 2020 [cited 2022 May 10]. https://www.cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medi...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

