Nivolumab and Relatlimab in Patients With Advanced Melanoma That Had Progressed on Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy: Results From the Phase I/IIa RELATIVITY-020 Trial
- PMID: 36780608
- PMCID: PMC10431305
- DOI: 10.1200/JCO.22.02072
Nivolumab and Relatlimab in Patients With Advanced Melanoma That Had Progressed on Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy: Results From the Phase I/IIa RELATIVITY-020 Trial
Abstract
Purpose: Nivolumab and relatlimab activity in advanced melanoma with prior progression on anti-programmed death-1/programmed death ligand 1 (PD-(L)1)-containing regimens is under investigation. RELATIVITY-047 demonstrated significantly improved progression-free survival (PFS) for nivolumab and relatlimab over nivolumab in previously untreated advanced melanoma.
Methods: The phase I/IIa, open-label RELATIVITY-020 trial part D assessed efficacy and safety of nivolumab and relatlimab in advanced melanoma with progression during, or within 3 months of, 1 (D1) or ≥ 1 (D2) anti-PD-(L)1-containing regimens. Safety was a primary end point. Objective response rate (coprimary end point) and PFS by blinded independent central review (BICR) were assessed.
Results: Five hundred eighteen patients (D1 = 354; D2 = 164) received nivolumab and relatlimab. Among evaluable patients, the objective response rate by BICR was 12.0% (95% CI, 8.8 to 15.8) in D1 (n = 351) and 9.2% (95% CI, 5.2 to 14.7) in D2 (n = 163). Responses appeared to be enriched among patients with tumors expressing programmed death ligand 1 or lymphocyte activation gene 3; however, responses were observed regardless of programmed death ligand 1 and lymphocyte activation gene 3 expression (1%). The median duration of response was not reached (95% CI, 12.9 to not reached) in D1 and 12.8 months (95% CI, 6.9 to 12.9) in D2. The median PFS by BICR was 2.1 months (95% CI, 1.9 to 3.5) in D1 and 3.2 months (95% CI, 1.9 to 3.6) in D2; the 6-month PFS rate was 29.1% (95% CI, 24.2 to 34.1) and 27.7% (95% CI, 20.5 to 35.4), respectively. The grade 3-4 treatment-related adverse event incidence was 15.0% in D1 and 12.8% in D2. One case of grade 3 myocarditis and no treatment-related deaths occurred across part D.
Conclusion: Nivolumab and relatlimab had a manageable safety profile and demonstrated durable clinical activity in a proportion of patients with heavily pretreated advanced melanoma with prior progression on anti-PD-(L)1-containing regimens.
[Media: see text].
Trial registration: ClinicalTrials.gov NCT01968109.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
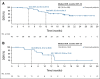
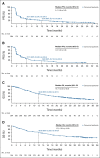
Comment in
-
Combination Immune Checkpoint Inhibition: Is First Line Best?J Clin Oncol. 2023 May 20;41(15):2691-2694. doi: 10.1200/JCO.23.00061. Epub 2023 Feb 28. J Clin Oncol. 2023. PMID: 36854080 No abstract available.
References
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 - PubMed
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol 39, 2021. (suppl 15; abstr 9506)
-
- Nordstrom BL, Hamilton M, Collins JM, et al. : Treatment patterns and outcomes following disease progression on anti-PD-1 therapies for advanced melanoma. Future Oncol 18:1343-1355, 2022 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

