Targeting ATP12A, a Nongastric Proton Pump α Subunit, for Idiopathic Pulmonary Fibrosis Treatment
- PMID: 36780662
- PMCID: PMC10257074
- DOI: 10.1165/rcmb.2022-0264OC
Targeting ATP12A, a Nongastric Proton Pump α Subunit, for Idiopathic Pulmonary Fibrosis Treatment
Abstract
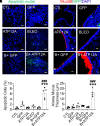
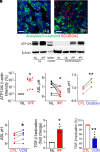
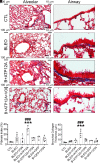
Idiopathic pulmonary fibrosis (IPF) is a pathological condition of unknown etiology that results from injury to the lung and an ensuing fibrotic response that leads to the thickening of the alveolar walls and obliteration of the alveolar space. The pathogenesis is not clear, and there are currently no effective therapies for IPF. Small airway disease and mucus accumulation are prominent features in IPF lungs, similar to cystic fibrosis lung disease. The ATP12A gene encodes the α-subunit of the nongastric H+, K+-ATPase, which functions to acidify the airway surface fluid and impairs mucociliary transport function in patients with cystic fibrosis. It is hypothesized that the ATP12A protein may play a role in the pathogenesis of IPF. The authors' studies demonstrate that ATP12A protein is overexpressed in distal small airways from the lungs of patients with IPF compared with normal human lungs. In addition, overexpression of the ATP12A protein in mouse lungs worsened bleomycin induced experimental pulmonary fibrosis. This was prevented by a potassium competitive proton pump blocker, vonoprazan. These data support the concept that the ATP12A protein plays an important role in the pathogenesis of lung fibrosis. Inhibition of the ATP12A protein has potential as a novel therapeutic strategy in IPF treatment.
Keywords: ATP12A; bleomycin; proton pump blocker; pulmonary fibrosis; small airways.
Figures




Comment in
-
ATP12A: Connecting Mucus and Fibrosis in Idiopathic Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2023 Jun;68(6):603-604. doi: 10.1165/rcmb.2023-0096ED. Am J Respir Cell Mol Biol. 2023. PMID: 36996473 Free PMC article. No abstract available.
References
-
- Pardo A, Selman M. The interplay of the genetic architecture, aging, and environmental factors in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol . 2021;64:163–172. - PubMed
-
- Fonseca C, Abraham D, Black CM. Lung fibrosis. Springer Semin Immunopathol . 1999;21:453–474. - PubMed
-
- King TE, Jr, Albera C, Bradford WZ, Costabel U, du Bois RM, Leff JA, et al. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med . 2014;189:825–831. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2071–2082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

