Hydrogen sulfide as an anti-calcification stratagem in human aortic valve: Altered biogenesis and mitochondrial metabolism of H2S lead to H2S deficiency in calcific aortic valve disease
- PMID: 36780769
- PMCID: PMC9947110
- DOI: 10.1016/j.redox.2023.102629
Hydrogen sulfide as an anti-calcification stratagem in human aortic valve: Altered biogenesis and mitochondrial metabolism of H2S lead to H2S deficiency in calcific aortic valve disease
Abstract
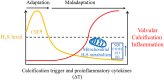
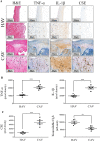
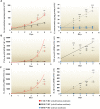
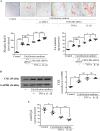
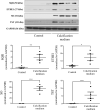
Hydrogen sulfide (H2S) was previously revealed to inhibit osteoblastic differentiation of valvular interstitial cells (VICs), a pathological feature in calcific aortic valve disease (CAVD). This study aimed to explore the metabolic control of H2S levels in human aortic valves. Lower levels of bioavailable H2S and higher levels of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were detected in aortic valves of CAVD patients compared to healthy individuals, accompanied by higher expression of cystathionine γ-lyase (CSE) and same expression of cystathionine β-synthase (CBS). Increased biogenesis of H2S by CSE was found in the aortic valves of CAVD patients which is supported by increased production of lanthionine. In accordance, healthy human aortic VICs mimic human pathology under calcifying conditions, as elevated CSE expression is associated with low levels of H2S. The expression of mitochondrial enzymes involved in H2S catabolism including sulfide quinone oxidoreductase (SQR), the key enzyme in mitochondrial H2S oxidation, persulfide dioxygenase (ETHE1), sulfite oxidase (SO) and thiosulfate sulfurtransferase (TST) were up-regulated in calcific aortic valve tissues, and a similar expression pattern was observed in response to high phosphate levels in VICs. AP39, a mitochondria-targeting H2S donor, rescued VICs from an osteoblastic phenotype switch and reduced the expression of IL-1β and TNF-α in VICs. Both pro-inflammatory cytokines aggravated calcification and osteoblastic differentiation of VICs derived from the calcific aortic valves. In contrast, IL-1β and TNF-α provided an early and transient inhibition of VICs calcification and osteoblastic differentiation in healthy cells and that effect was lost as H2S levels decreased. The benefit was mediated via CSE induction and H2S generation. We conclude that decreased levels of bioavailable H2S in human calcific aortic valves result from an increased H2S metabolism that facilitates the development of CAVD. CSE/H2S represent a pathway that reverses the action of calcifying stimuli.
Keywords: Arteriosclerosis; Chronic kidney disease; Hydrogen sulfide; Mitochondrial H(2)S catabolism; Phosphate; Valvular inflammation; Vascular calcification.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest All the authors declared no competing interests. MW has patent applications for the therapeutic use of slow hydrogen sulfide donor molecules.
Figures







References
-
- O'Brien K.D., et al. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92(8):2163–2168. - PubMed
-
- Mohler E.R., 3rd, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. - PubMed
-
- Freeman R.V., Otto C.M. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111(24):3316–3326. - PubMed
-
- Rajamannan N.M., et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–1791. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

