Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study
- PMID: 36780912
- PMCID: PMC10014040
- DOI: 10.1016/S1473-3099(23)00011-7
Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study
Abstract
Background: Nirmatrelvir is a protease inhibitor with in-vitro activity against SARS-CoV-2, and ritonavir-boosted nirmatrelvir can reduce the risk of progression to severe COVID-19 among individuals at high risk infected with delta and early omicron variants. However, less is known about the effectiveness of nirmatrelvir-ritonavir during more recent BA.2, BA2.12.1, BA.4, and BA.5 omicron variant surges. We used our real-world data platform to evaluate the effect of nirmatrelvir-ritonavir treatment on 28-day hospitalisation, mortality, and emergency department visits among outpatients with early symptomatic COVID-19 during a SARS-CoV-2 omicron (BA.2, BA2.12.1, BA.4, and BA.5) predominant period in Colorado, USA.
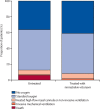
Methods: We did a propensity-matched, retrospective, observational cohort study of non-hospitalised adult patients infected with SARS-CoV-2 between March 26 and Aug 25, 2022, using records from a statewide health system in Colorado. We obtained data from the electronic health records of University of Colorado Health, the largest health system in Colorado, with 13 hospitals and 141 000 annual hospital admissions, and with numerous ambulatory sites and affiliated pharmacies around the state. Included patients had a positive SARS-CoV-2 test or nirmatrelvir-ritonavir medication order. Exclusion criteria were an order for or administration of other SARS-CoV-2 treatments within 10 days of a positive SARS-CoV-2 test, hospitalisation at the time of positive SARS-CoV-2 test, and positive SARS-CoV-2 test more than 10 days before a nirmatrelvir-ritonavir order. We propensity score matched patients treated with nirmatrelvir-ritonavir with untreated patients. The primary outcome was 28-day all-cause hospitalisation.
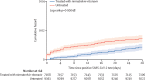
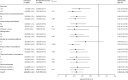
Findings: Among 28 167 patients infected with SARS-CoV-2 between March 26 and Aug 25, 2022, 21 493 met the study inclusion criteria. 9881 patients received treatment with nirmatrelvir-ritonavir and 11 612 were untreated. Nirmatrelvir-ritonavir treatment was associated with reduced 28-day all-cause hospitalisation compared with no antiviral treatment (61 [0·9%] of 7168 patients vs 135 [1·4%] of 9361 patients, adjusted odds ratio (OR) 0·45 [95% CI 0·33-0·62]; p<0·0001). Nirmatrelvir-ritonavir treatment was also associated with reduced 28-day all-cause mortality (two [<0·1%] of 7168 patients vs 15 [0·2%] of 9361 patients; adjusted OR 0·15 [95% CI 0·03-0·50]; p=0·0010). Using subsequent emergency department visits as a surrogate for clinically significant relapse, we observed a decrease after nirmatrelvir-ritonavir treatment (283 [3·9%] of 7168 patients vs 437 [4·7%] of 9361 patients; adjusted OR 0·74 [95% CI 0·63-0·87]; p=0·0002).
Interpretation: Real-world evidence reported during a BA.2, BA2.12.1, BA.4, and BA.5 omicron surge showed an association between nirmatrelvir-ritonavir treatment and reduced 28-day all-cause hospitalisation, all-cause mortality, and visits to the emergency department. With results that are among the first to suggest effectiveness of nirmatrelvir-ritonavir for non-hospitalised patients during an omicron period inclusive of BA.4 and BA.5 subvariants, these data support nirmatrelvir-ritonavir as an ongoing first-line treatment for adults acutely infected with SARS-CoV-2.
Funding: US National Institutes of Health.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NRA reports grants from the US National Institutes of Health (NIH), during the conduct of the study. KCM reports grants from the National Center for Advancing Translational Sciences (NCATS), during the conduct of the study, and grants from the National Institute of Child Health and Human Development (NICHD) and the National Heart, Lung, and Blood Institute (NHLBI), outside of the submitted work. TDB reports grants from the NCATS, during the conduct of the study, and grants from the NICHD and NHLBI, outside of the submitted work. NEC reports grants from the US NIH, during the conduct of the study. AAG reports grants from the US NIH during the conduct of the study, grants from the US Centers for Disease Control, the US Department of Defense, AbbVie, and Faron Pharmaceuticals, and participation on an NIH data safety monitoring board, outside of the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Real-world effectiveness of nirmatrelvir-ritonavir against BA.4 and BA.5 omicron SARS-CoV-2 variants.Lancet Infect Dis. 2023 Jun;23(6):639-640. doi: 10.1016/S1473-3099(23)00056-7. Epub 2023 Feb 10. Lancet Infect Dis. 2023. PMID: 36780911 Free PMC article. No abstract available.
References
-
- Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. - PubMed
-
- US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for Paxlovid. https://www.fda.gov/media/155050/download
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

