Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022
- PMID: 36780914
- PMCID: PMC9917454
- DOI: 10.1016/S2213-2600(23)00015-2
Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022
Abstract
Background: Synthesising evidence on the long-term vaccine effectiveness of COVID-19 vaccines (BNT162b2 [Pfizer-BioNTech], mRNA-1273 [Moderna], ChAdOx1 nCoV-19 [AZD1222; Oxford-AstraZeneca], and Ad26.COV2.S [Janssen]) against infections, hospitalisations, and mortality is crucial to making evidence-based pandemic policy decisions.
Methods: In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose. Single reviewers assessed titles, abstracts, and full-text articles, and extracted data, with a second reviewer verifying included studies. The primary outcomes were vaccine effectiveness against SARS-CoV-2 infections, hospitalisations, and mortality, which were assessed using three-level meta-analytic models. This study is registered with the National Collaborating Centre for Methods and Tools, review 473.
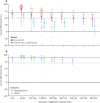
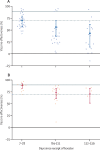
Findings: We screened 16 696 records at the title and abstract level, appraised 832 (5·0%) full texts, and initially included 73 (0·4%) studies. Of these, we excluded five (7%) studies because of critical risk of bias, leaving 68 (93%) studies that were extracted for analysis. For infections caused by any SARS-CoV-2 strain, vaccine effectiveness for the primary series reduced from 83% (95% CI 80-86) at baseline (14-42 days) to 62% (53-69) by 112-139 days. Vaccine effectiveness at baseline was 92% (88-94) for hospitalisations and 91% (85-95) for mortality, and reduced to 79% (65-87) at 224-251 days for hospitalisations and 86% (73-93) at 168-195 days for mortality. Estimated vaccine effectiveness was lower for the omicron variant for infections, hospitalisations, and mortality at baseline compared with that of other variants, but subsequent reductions occurred at a similar rate across variants. For booster doses, which covered mostly omicron studies, vaccine effectiveness at baseline was 70% (56-80) against infections and 89% (82-93) against hospitalisations, and reduced to 43% (14-62) against infections and 71% (51-83) against hospitalisations at 112 days or later. Not enough studies were available to report on booster vaccine effectiveness against mortality.
Interpretation: Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Funding: Canadian Institutes of Health Research and the Public Health Agency of Canada.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests In the past 3 years, SLB has received consultancy and speaker fees from Resplipus, an investigator-generated educational grant from Moderna, and has served on advisory boards for Bayer, Sanofi, Respiplus, and Sojecci, none of which are related to the current Article. All other authors declare no competing interests.
Figures
Comment in
-
COVID-19 vaccine effectiveness and evolving variants: understanding the immunological footprint.Lancet Respir Med. 2023 May;11(5):395-396. doi: 10.1016/S2213-2600(23)00140-6. Epub 2023 Apr 17. Lancet Respir Med. 2023. PMID: 37080227 Free PMC article. No abstract available.
References
-
- WHO . World Health Organization; 2021. Vaccine efficacy, effectiveness and protection.https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-ef...
-
- Bacon S, Wu N, Joyal-Desmarais K, et al. What is the long-term effectiveness of available VID-19 vaccines for adults, including for variants of concern and over time frames beyond 112 days in those with a primary series and beyond 84 days in those with a primary series and an additional dose? McMaster Health Forum. 2022. https://www.mcmasterforum.org/networks/covid-end/resources-specific-to-c...
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

