A Single Dose, Thermostable, Trivalent Human Papillomavirus Vaccine Formulated Using Atomic Layer Deposition
- PMID: 36780987
- PMCID: PMC10363232
- DOI: 10.1016/j.xphs.2023.02.007
A Single Dose, Thermostable, Trivalent Human Papillomavirus Vaccine Formulated Using Atomic Layer Deposition
Abstract
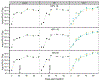
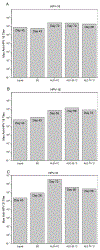
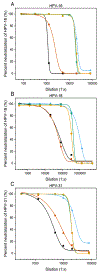
Formulations of human papillomavirus (HPV) 16, 18, and 31 L1 capsomere protein antigens were spray dried to obtain glassy microspheres that were then coated by atomic layer deposition (ALD) with nanometer-thin protective layers of alumina. Spray-drying was used to formulate human papillomavirus (HPV) 16, 18, and 31 L1 capsomere protein antigens within glassy microspheres to which nanoscopic protective layers of alumina were applied using ALD. Suspensions of alumina-coated, capsomere-containing microparticles were administered in a single dose to mice. ALD-deposited alumina coatings provided thermostability and a delayed in vivo release of capsomere antigens, incorporating both a prime and a boost dose in one injection. Total serotype-specific antibody titers as well as neutralizing titers determined from pseudovirus infectivity assays were unaffected by incubation of the ALD-coated vaccines for at 4, 50, or 70 °C for three months prior to administration. In addition, even after incubation for three months at 70 °C, single doses of ALD-coated vaccines produced both higher total antibody responses and higher neutralizing responses than control immunizations that used two doses of conventional liquid formulations stored at 4 °C.
Keywords: Atomic layer deposition; Controlled release; Human papilloma virus vaccine; Thermostability.
Copyright © 2023 American Pharmacists Association. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: TWR and RLG are inventors of relevant intellectual property owned by the Regents of the University of Colorado and licensed to Vitrivax, Inc., a company in which they hold financial interest.
Figures
References
-
- Pingali C; Yankey D; Elam-Evans LD; Markowitz LE; Williams CL; Fredua B; McNamara LA; Stokley S; Singleton JA National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020. MMWR Morb Mortal Wkly Rep 2021, 70 (35), 1183–1190. DOI:10.15585/mmwr.mm7035a1 From NLM Medline. - DOI - PMC - PubMed
-
- Rosenblum HG; Lewis RM; Gargano JW; Querec TD; Unger ER; Markowitz LE Declines in Prevalence of Human Papillomavirus Vaccine-Type Infection Among Females after Introduction of Vaccine - United States, 2003-2018. MMWR Morb Mortal Wkly Rep 2021, 70 (12), 415–420. DOI:10.15585/mmwr.mm7012a2 From NLM Medline. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

