Human Ductal Carcinoma In Situ: Advances and Future Perspectives
- PMID: 36781223
- PMCID: PMC10547390
- DOI: 10.1101/cshperspect.a041319
Human Ductal Carcinoma In Situ: Advances and Future Perspectives
Abstract
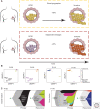
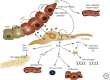
Due to widespread adoption of screening mammography, there has been a significant increase in new diagnoses of ductal carcinoma in situ (DCIS). However, DCIS outcomes remain unclear. A large fraction of human DCIS (>50%) may not need the multimodality treatment options currently offered to all DCIS patients. More importantly, while we may be overtreating many, we cannot identify those most at risk of invasion or metastasis following a DCIS diagnosis. This review summarizes the studies that have furthered our understanding of DCIS pathology and mechanisms of invasive progression by using advanced technologies including spatial genomics, transcriptomics, and multiplex proteomics. This review also highlights a need for rethinking DCIS with a more focused view on epithelial states and programs and their cross talk with the microenvironment.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Barcellos-Hoff MH, Ravani SA. 2000. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res 60: 1254–1260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
