Clinical relevance of PD-1 positive CD8 T-cells in gastric cancer
- PMID: 36781556
- PMCID: PMC10115710
- DOI: 10.1007/s10120-023-01364-7
Clinical relevance of PD-1 positive CD8 T-cells in gastric cancer
Abstract
Background: We evaluated the relevance of PD-1+CD8+ T-cells in gastric cancer (GC) including prognostic significance, association with chemotherapy and immunotherapy sensitivity and correlations with the tumor microenvironment (TME).
Methods: Discovery cohort: GC samples were evaluated for AE1/3, CD8, PD-1, Ki-67 and Granzyme-B expression with fluorescence-based multiplex immunohistochemistry (mIHC). Validation cohorts: we analyzed bulk RNAseq GC datasets from TCGA, the "3G" chemotherapy trial and an immunotherapy phase 2 trial. The cox proportional hazards model was used to identify factors that influenced overall survival (OS). To study the TME, we analyzed single-cell RNAseq performed on GCs.
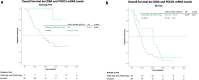
Results: In the discovery cohort of 350 GCs, increased PD-1 expression of CD8 T-cells was prognostic for OS (HR 0.822, p = 0.042). PD-1 expression in CD8 T-cells highly correlated with cytolytic [Granzyme-B+] (r = 0.714, p < 0.001) and proliferative [Ki-67+] (r = 0.798, p < 0.001) activity. Analysis of bulk RNAseq datasets showed tumors with high PD-1 and CD8A expression levels had improved OS when treated with immunotherapy (HR 0.117, p = 0.036) and chemotherapy (HR 0.475, p = 0.017). Analysis of an scRNAseq dataset of 152,423 cells from 40 GCs revealed that T-cell and NK-cell proportions were higher (24% vs 18% and 19% vs 15%, p < 0.0001), while macrophage proportions were lower (7% vs 11%, p < 0.0001) in CD8PD-1high compared to CD8PD-1low tumors.
Conclusion: This is one of the largest GC cohorts of mIHC combined with analysis of multiple datasets providing orthogonal validation of the clinical relevance of PD-1+CD8+ T-cells being associated with improved OS. CD8PD-1high tumors have distinct features of an immunologically active, T-cell inflamed TME.
Keywords: CD8 T-cells; Gastric cancer; Multiplex immunohistochemistry; PD-1.
© 2023. The Author(s).
Conflict of interest statement
WPY has received Honoraria and consulting fees from Abbvie/Genentech, Amgen, AstraZeneca, Bristol Myers Squibb, Ipsen, Novartis, Bayer, Eisai, Lilly, MSD, Sanofi/Aventis and Taiho Pharmaceutical. RS has received Honoraria from Bristol Myers Squibb, MSD, Lilly, Roche, Taiho Pharmaceutical, AstraZeneca and DKSH; consulting fees from Bristol Myers Squibb, Eisai, Taiho Pharmaceutical, Bayer, Merck, Novartis and MSD; research funding from Paxman and MSD; travel, accommodation funding support from Roche, AstraZeneca, Taiho Pharmaceutical and Eisai. PT has stock and other ownership interests in HealthSeq, research funding from Kyowa Hakko Kirin and Thermo Fisher Scientific, and patents/other intellectual property through the Agency for Science and Technology Research, Singapore (all outside the submitted work). CEC has received Honoraria and consulting fees from AstraZeneca, Roche/Genentech and Guardant Health AMEA; travel and accommodation support from Taiho Pharmaceutical. JC, LFK, MYS, BRA, BKJT, CBT, RYKT, JS, AS, KG, HLT, GC, HM, GKR, JHL, SS declared no conflicts of interest.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians.n/a(n/a). - PubMed
-
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. - DOI - PubMed
-
- Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370. - DOI - PMC - PubMed
-
- Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2018;392(10142):123–133. doi: 10.1016/S0140-6736(18)31257-1. - DOI - PubMed
-
- Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low Programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2022;40(4):392–402. doi: 10.1200/JCO.21.01862. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

