Anti-inflammatory Action of BT75, a Novel RARα Agonist, in Cultured Microglia and in an Experimental Mouse Model of Alzheimer's Disease
- PMID: 36781685
- PMCID: PMC10355192
- DOI: 10.1007/s11064-023-03888-x
Anti-inflammatory Action of BT75, a Novel RARα Agonist, in Cultured Microglia and in an Experimental Mouse Model of Alzheimer's Disease
Abstract
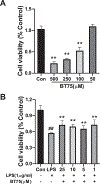
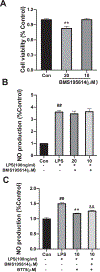
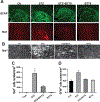
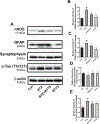
BT75, a boron-containing retinoid, is a novel retinoic acid receptor (RAR)α agonist synthesized by our group. Previous studies indicated that activation of retinoic acid (RA) signaling may attenuate progression of Alzheimer's disease (AD). Presently, we aimed to examine the anti-inflammatory effect of BT75 and explore the possible mechanism using cultured cells and an AD mouse model. Pretreatment with BT75 (1-25 µM) suppressed the release of nitric oxide (NO) and IL-1β in the culture medium of mouse microglial SIM-A9 cells activated by LPS. BMS195614, an RARα antagonist, partially blocked the inhibition of NO production by BT75. Moreover, BT75 attenuated phospho-Akt and phospho-NF-κB p65 expression augmented by LPS. In addition, BT75 elevated arginase 1, IL-10, and CD206, and inhibited inducible nitric oxide synthase (iNOS) and IL-6 formation in LPS-treated SIM-A9 cells, suggesting the promotion of M1-M2 microglial phenotypic polarization. C57BL/6 mice were injected intracerebroventricularly (icv) with streptozotocin (STZ) (3 mg/kg) to provide an AD-like mouse model. BT75 (5 mg/kg) or the vehicle was intraperitoneally (ip) injected to icv-STZ mice once a day for 3 weeks. Immunohistochemical analyses indicated that GFAP-positive cells and rod or amoeboid-like Iba1-positive cells, which increased in the hippocampal fimbria of icv-STZ mice, were reduced by BT75 treatment. Western blot results showed that BT75 decreased levels of neuronal nitric oxide synthase (nNOS), GFAP, and phosphorylated Tau, and increased levels of synaptophysin in the hippocampus of icv-STZ mice. BT75 may attenuate neuroinflammation by affecting the Akt/NF-κB pathway and microglial M1-M2 polarization in LPS-stimulated SIM-A9 cells. BT75 also reduced AD-like pathology including glial activation in the icv-STZ mice. Thus, BT75 may be a promising anti-inflammatory and neuroprotective agent worthy of further AD studies.
Keywords: Boron-containing retinoid; Microglial polarization; Neurodegenerative disease; Neuroinflammation; Nitric oxide; Retinoic acid receptor.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures


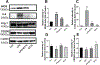

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

